Abstract
Gene expression of neuronal nitric oxide synthase (nNOS) changes in the hypothalamic paraventricular nucleus (PVN) depending on feeding conditions, which is decreased during food deprivation and restored by refeeding, and phosphorylated cAMP response element binding protein (pCREB) was suggested to play a role in its regulation. This study was conducted to examine if the fasting-induced down-regulation of the PVN-nNOS expression is restored by activation of cAMP-dependent protein kinase A (cAMP/PKA) pathway. Freely moving rats received intracerebroventricular (icv) injection of cAMP/PKA activator Sp-cAMP (40 nmol) or vehicle (sterilized saline) following 48 h of food deprivation. One hour after drug injections, rats were transcardially perfused with 4% paraformaldehyde, and the PVN tissues were processed for nNOS or pCREB immunohistochemistry. Sp-cAMP significantly increased not only nNOS but also pCREB immunoreactivities in the PVN of food deprived rats. Fasting-induced down-regulation of the PVN-nNOS was restored by 1 h after the icv Sp-cAMP. Results suggest that cAMP/PKA pathway may mediate the regulation of the PVN-nNOS expression depending on different feeding conditions.
Food deprivation decreases mRNA level of neuronal nitric oxide synthase (nNOS), the intracellular intensity of NADPH-diaphorase (NADPH-d) staining, a marker for nNOS activity in the brain, and the apparent number of nNOS immunoreactive cells in the hypothalamic paraventricular nucleus (PVN) [1-5]. Plasma glucocorticoids, which are elevated during food deprivation [3,5-7], appear to mediate the fasting-induced down-regulation of nNOS in the hypothalamic PVN. We have reported that depletion of endogenous glucocorticoids by adrenalectomy abolishes fasting-induced down-regulation of nNOS in the rat PVN, and dexamethasone decreases the PVN-nNOS in both adrenalectomized and intact rats [4,8]. Furthermore, treatment with a glucocorticoids receptor antagonist RU486 during food deprivation blunted the fasting-induced decrease of nNOS in the PVN [3]. These reports together suggested that plasma glucocorticoids may suppress nNOS expression in the PVN during food deprivation, via its receptor-mediated pathway.
However, the upstream promoter of nNOS does not carry glucocorticoid response element, but carries cAMP response element [9-11]. In vitro studies have reported that nNOS expression is regulated by a cAMP response element-binding protein (CREB) family transcription factor-dependent mechanism [11], and that phosphorylation of Ser-133 residue in CREB, a critical step in the activation of CREB, is regulated by glucocorticoids [12,13]. We have reported that CREB phosphorylation in the PVN is markedly decreased by 48 h of food deprivation, and this reduction is inhibited by treatment with a glucocorticoid receptor antagonist RU486 [3]. Thus, it is suggested that the plasma glucocorticoids may suppress nNOS expression in the PVN during food deprivation, via down-regulation of CREB phosphorylation.
Not only the fasting-induced decrease of the PVN-nNOS but also of the PVN-pCREB is restored shortly after refeeding [5], and the refeeding-induced increases of the hypothalamic CREB phosphorylation and nNOS expression are blocked by dexamethasone treatments [5,14]. Also, the fasting-induced elevation of the plasma glucocorticoids is restored shortly after refeeding [5,15]. These reports together suggest that refeeding-induced nNOS expression and CREB phosphorylation in the PVN may require glucocorticoid dis-inhibition. However, our previous study demonstrated that glucocorticoid dis-inhibition alone is not sufficient to induce nNOS expression in the PVN [5]. It was reported that food deprivation decreases the hypothalamic cAMP levels and refeeding normalizes it [16]. In this study, we demonstrate that the intracerebroventricular (icv) injection of the membrane-permeable cAMP-dependent protein kinase A (PKA) activator Sp-cAMP (adenosine-3',5'-cyclic monophosphorothioate Sp-isomer) without food supplement rescues the fasting-induced decrease of nNOS expression in the PVN without affecting the plasma corticosterone levels.
Sprague-Dawley rats were purchased (Samtako Bio, Osan, Korea), and cared in a specific-pathogen-free (SPF) barrier area with constant control of temperature (22±1℃), humidity (55%), and a 12/12 hr light/dark cycle (lights-on at 07:00 AM). Standard laboratory food (Purina Rodent Chow, Purina Co., Seoul, Korea) and membrane filtered purified water were available ad libitum. Animals were cared according to the Guideline for Animal Experiments, 2000, edited by the Korean Academy of Medical Sciences, which is consistent with the NIH Guidelines for the Care and Use of Laboratory Animals, revised 1996. All animal experiments were approved by the Committee for the Care and Use of Laboratory Animals at Seoul National University.
Under chloral hydrate (153 mg/kg) and pentobarbital (35 mg/kg) anesthesia, rats (280~300 g) were stereotaxically implanted with a 22-gauge, stainless steel guide cannula (Plastics One, Roanoke, VA) aimed towards the lateral ventricle (1.2 mm caudal to bregma, 1.5 mm lateral to the midline, and 4 mm below the skull surface). Guide cannulae were held in place with dental acrylic bonded to stainless steel screws anchored to the skull. An obdurator was inserted into each guide cannula and remained in place except during injections when it was removed and replaced with an injector that extended 1.0 mm beyond the tip of the guide cannula. After 1 week of post-operational recovery, patency and placement of the cannula was verified by injection of 100 ng human angiotensin II (Sigma Chemical Co., St Louis, MO, USA) dissolved in 5 µl of 0.15 M NaCl; rats with cannulas projecting into the lateral ventricle responded to the angiotensin injection by vigorously licking the water bottle within 2 min [17-19], while rats that failed to drink were dropped from the study. Cannula placements were also verified postmortem by sectioning through the brain.
Rats verified for the cannula placement were divided into four different groups (n=6 in each group, total 24 rats); i.e, FC/vehicle (saline icv with ad libitum access to food), FD/vehicle (saline icv following food deprivation), FD/Sp-cAMP (Sp-cAMP icv following food deprivation) and FD/Rp-cAMP (Rp-cAMP icv following food deprivation). Rats in FD/vehicle, FD/Sp-cAMP or FD/Rp-cAMP groups underwent a 48 h of food deprivation beginning at 09:00 h, and then received an icv injection of Sp-cAMP (Sp-8-bromo-cAMP, Sigma Chemical Co., St Louis, MO, USA; 5 µl of 40 nmol in sterilized saline), Rp-cAMP (Rp-8-bromo-cAMP, Sigma Chemical Co., St Louis, MO, USA; 5 µl of 40 nmol in sterilized saline) or sterilized saline (5 µl of 0.15 M NaCl). Sp-cAMP, Rp-cAMP or saline was delivered through the icv guide cannulas over 30 s with a handheld 50 µl syringe (Hamilton Co., Reno, NV, USA). The injector was left in place for 30 s after solution delivery. Free-fed control group (FC/vehicle) received an icv injection of sterilized saline (5 µl of 0.15 M NaCl).
One hour after the drug injections, rats were anesthetized with over doses of sodium pentobarbital (Hallym Pharmaceutical Co., Seoul, Korea), and transcardiac perfusions were performed first with heparinized isotonic saline then with ice-cold 4% paraformaldehyde (Sigma Co., MO, USA) in 0.1 M sodium phosphate buffer. Brains were immediately dissected out, blocked, post-fixed for 2 h, and transferred into 30% sucrose (Sigma Co., MO, USA) for cryoprotection. Forty-micron coronal sections were cut on a freezing, sliding microtome (HM440E, Microm Co., Germany). Alternate sections were collected throughout the rostrocaudal extent of the hypothalamic PVN (between bregma - 1.3 mm and - 2.1 mm) [20]. Immunohistochemistry was performed with standard DAB reaction using commercial ABC kit (Vectastain Elite Kit, Vector Laboratories, CA, USA) as previously described [21]. Polyclonal rabbit anti-nNOS, which reacts with the 160 kDa nNOS protein and does not exhibit any cross-reactivity with the related eNOS or iNOS proteins (1:2,000 dilution, Zymed Laboratories, CA, USA), and anti-pCREB, which recognizes p43 phosphorylated CREB (1:1,000 dilution, Upstate biotechnology, NY, USA), antibodies were used as primary antibodies, and biotinylated anti-rabbit IgG (1:200 dilution, Vector Laboratories, CA, USA) as secondary. Immuno-stained sections were mounted in an anatomical order onto gelatin-coated slides from 0.05 M phosphate buffer, air-dried, dehydrated through a graded ethanol to xylene, and cover-slipped.
Cardiac blood was rapidly collected in the heparinized tube immediately after exposing the heart for perfusion, and centrifuged at 2,000 rpm for 20 min. The plasma was transferred into new tubes, frozen in liquid nitrogen, and stored at -80℃ until used for the assay. Plasma corticosterone levels were determined by radioimmunoassay using 125I-labelled Coat-A-Count kit (DPC, CA, USA). All rats were sacrificed between 10:00 h and 12:00 h to minimize diurnal variation in the plasma levels of corticosterone [22].
The number of nNOS immunopositive cells was blind-counted by hand, pCREB immunopositive nuclei autocounted, after digitizing 720×540 micron images of three sections from the PVN (closest sections to bregma - 1.88 mm) from each brain using an Olympus BX-50 microscope (Olympus Co., Tokyo, Japan) and MCID image analysis system (M2, Imaging Research Inc., Ontario, Canada). nNOS manual count was done blind by S.B.Y. and confirmed by a separate blind count by J.Y.L. Cell counts from the sections of each rat were averaged per section, and the individual mean counts averaged across rats within experimental groups. All data were analyzed by one-way analysis of variance (ANOVA), and preplanned comparisons with the control performed by post-hoc Fisher's PLSD and presented by means±SE. For all comparisons, the level of significance was set at p≤0.05.
Food deprivation significantly reduced the number of nNOS immuno-positive (-ir) neurons in both the medial parvocellular (mP) and the posterior magnocellular (pM) PVN (Fig. 1). One hour after an icv Sp-cAMP, nNOS-ir was significantly increased in the parvocellular PVN of food deprived rats (p<0.05, FD/veh vs. FD/Sp-cAMP); however, this increase was not observed in the magnocellular PVN. nNOS-ir in the PVN of food deprived rats that received an icv Rp-cAMP, cAMP antagonist, did not differ from the food deprived rats that received icv saline.
pCREB-ir in the parvocellular PVN of food deprived rats that received icv Sp-cAMP was increased significantly (p<0.05) compared with vehicle injected controls (Fig. 2). Sp-cAMP did not increase pCREB-ir in the magnocellular PVN of food deprived rats.
Cardiac blood was collected at sacrifice and the plasma levels of corticosterone were analyzed by radioimmunoassay (Fig. 3). Food deprivation significantly increased the plasma levels of corticosterone (p<0.05, FC/veh vs. FD/veh), and Sp-cAMP administration did not alter the plasma corticosterone levels of food deprived rats.
We have previously demonstrated that nNOS gene expression in the hypothalamic PVN changes depending on feeding conditions; i.e., it is decreased during food deprivation and restored following refeeding, and that pCREB may be involved in the PVN-nNOS expression [3,5]. The upstream promoter of nNOS gene contains cAMP response element (CRE) [9]. In vitro studies have reported that nNOS expression is regulated by calcium influx through a CREB family transcription factor-dependent mechanism [11], and that cAMP markedly induces nNOS expression in human A673 neuroepithelial cells expressing CREB, and the induction is enhanced in A673 cells transfected with CREB [23]. In vivo study showed that the hypothalamic cAMP levels are decreased during food deprivation and restored by food consumption [16]. In this study, icv administration of cAMP-dependent PKA activator Sp-cAMP increased not only pCREB levels but also nNOS expression in the PVN of food deprived rats, and cAMP antagonist/PKA inhibitor Rp-cAMP [16] did not affect the fasting-induced down-regulation of the PVN-nNOS. Together, it is suggested that the regulation of the PVN-nNOS expression depending on feeding conditions may be mediated by cAMP/PKA pathway.
Previous studies have suggested that the plasma glucocorticoids may suppress nNOS expression in the PVN during food deprivation, via down-regulation of CREB phosphorylation [3,4,8], and that glucocorticoids dis-inhibition is required, although it is not sufficient, to restore the fasting-induced down-regulation of the PVN-nNOS and -pCREB [5,14]. However, in this study, Sp-cAMP administration increased both nNOS and pCREB in the PVN of food deprived rats, despite the fasting-induced elevation of the plasma corticosterone was still persisted. In vitro studies have reported that glucocorticoids regulate phosphorylation of Ser-133 residue in CREB, a critical step in activation of CREB [12,13], and interfere with the cAMP-induced DNA binding of CREB [24]. Underlying mechanism by which Sp-cAMP increased pCREB and nNOS in the PVN of food deprived rats despite of the glucocorticoids inhibition is currently not clear. It should be noticed that cAMP agonist used in this study Sp-cAMP is a more potent PKA activator with higher affinity to the regulatory subunit of PKA and more resistant to phosphodiesterase than endogenous cAMP [16,25], which might have allowed Sp-cAMP to overcome the glucocorticoids inhibition.
Our previous study suggested that refeeding-induced nNOS expression in the PVN is a nutrient directed event; i.e., refeeding with non-caloric palatable food mash failed to restore the fasting-induced decreases not only of the PVN-nNOS but also of -pCREB [5]. Many studies have examined the effects of various nutrients on NOS expression, however, those studies have been mostly focused on endothelial or inducible NOS, but not nNOS [26]. In vitro studies have demonstrated that glucose, the major substrate for cerebral energy metabolism, increases the intracellular cAMP content [27], and activates cAMP/PKA pathway [28]. Also, an intragastric infusion of glucose induced c-Fos expression, conventional marker for neuronal activation, in the PVN of food deprived rats [29]. Together with the current results, it is suggested that postprandial glucose may induce nNOS expression in the PVN at refeeding following food deprivation, by activating the cAMP/PKA pathway.
In this study, food deprivation down-regulated nNOS expression both in the parvocelluar and the magnocellular divisions of PVN; however, Sp-cAMP restored nNOS expression only in the parvocelluar PVN, but not in the magnocellular PVN. Furtheremore, Sp-cAMP following food deprivation increased pCREB levels only in the parvocellular PVN, but not in the magnocellular PVN. Our previous study showed that CREB phosphorylation is associated with nNOS down-regulation in the parvocellular PVN, but not in the magnocellular PVN, during food deprivation. That is, the fasting-induced nNOS down-regulation was observed not only in the parvocellular PVN but also in the magnocellular PVN; however, food deprivation reduced pCREB only in the parvocellular PVN [3]. Thus, it is concluded that nNOS expression in the magnocellular PVN, not likely in the parvocellular PVN, may not be mediated by cAMP/PKA pathway.
Nitric oxide (NO) is an important biological messenger that acts in a vast array of physiological processes [30]. In the nervous system, neuronal nitric oxide synthase (nNOS) accounts for the majority of the physiologic actions of NO [31,32]. Many investigations have shown that nNOS expression is dynamically regulated by both physiological and pathophysiological stimuli [31,33-35]. Feeding-related changes of nNOS expression in the PVN may be a part of the regulatory pathway for autonomic changes associated with feeding status, such as heart rate and blood pressure [36]. The PVN nitric oxide has been suggested to be involved in the regulation of autonomic functions [37,38], and reported to modulate vasopressin release [39]. Vasopressin is known to be involved in the control of blood pressure and heart rate as well as in the regulation of fluid and electrolyte balance [40]. Food deprivation decreases the hypothalamic contents of vasopressin [41] and refeeding activates vasopressin neurons in the PVN [15]. Overall, it is postulated that feeding-related changes in nNOS expression in the PVN may play a role in the regulatory control for blood pressure and heart rate, at least partly by modulating the activity of vasopressin neurons.
Figures and Tables
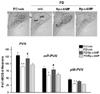 | Fig. 1nNOS immunoreactivity and number of nNOS-ir neurons in the parvocellular and the magnocellular PVN. Rats were sacrificed for nNOS immunohistochemistry at 1 h after an intracerebroventricular (icv) injection of Sp-cAMP or saline vehicle. FC/veh, free-fed control rats received icv administration of 5 µl sterilized saline; FD/veh, 48 h food deprivation and received icv administration of 5 µl sterilized saline; FD/Sp-cAMP, 48 h food deprivation and received icv administration of 5 µl of 40 nmol Sp-cAMP in sterilized saline; FD/Rp-cAMP, 48 h food deprivation and received icv administration of 5 µl of 40 nmol Rp-cAMP in sterilized saline; mP, medial parvocellular subdivision; pM, posterior magnocellular subdivision; PVN, paraventricular nucleus; 3v, third ventricle; *p<0.05, **p<0.01 vs. FC/veh; #p<0.05 vs. FD/veh, Values are presented as means±S.E. |
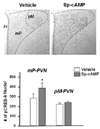 | Fig. 2pCREB immunoreactivity and number of pCREB-ir nuclei in the parvocellular and the magnocellular PVN. Rats were food deprived for 48 h and then received icv administration of 5 µl sterilized saline (vehicle) or 40 nmol Sp-cAMP in sterilized saline, and sacrificed 1 h after the icv injections for pCREB immunohistochemistry. mP, medial parvocellular subdivision; pM, posterior magnocellular subdivision; PVN, paraventricular nucleus; 3v, third ventricle; *p<0.05 vs. vehicle controls; Values are presented as means±S.E. |
 | Fig. 3Plasma corticosterone levels at 1 h after an icv administration of Sp-cAMP or saline vehicle. FC/veh, free-fed control rats received icv administration of 5 µl sterilized saline; FD/veh, 48 h food deprivation and received icv administration of 5 µl sterilized saline; FD/Sp-cAMP, 48 h food deprivation and received icv administration of 5 µl of 40 nmol Sp-cAMP in sterilized saline; *p<0.05 vs. FC/veh, Values are presented as means±S.E. |
ACKNOWLEDGEMENTS
This study was supported by grants from the Brain Research Center of the 21st Century Frontier Research Program (2012K001115) and the National Research Foundation (2010-0003642) funded by the Korea Government (Ministry of Education, Science and Technology).
References
1. Ueta Y, Levy A, Chowdrey HS, Lightman SL. Inhibition of hypothalamic nitric oxide synthase gene expression in the rat paraventricular nucleus by food deprivation is independent of serotonin depletion. J Neuroendocrinol. 1995. 7:861–865.
2. O'Shea RD, Gundlach AL. Food or water deprivation modulate nitric oxide synthase (NOS) activity and gene expression in rat hypothalamic neurones: correlation with neurosecretory activity? J Neuroendocrinol. 1996. 8:417–425.
3. Kim YM, Lee JY, Choi SH, Kim DG, Jahng JW. RU486 blocks fasting-induced decrease of neuronal nitric oxide synthase in the rat paraventricular nucleus. Brain Res. 2004. 1018:221–226.
4. Jahng JW, Lee JH, Kim GT, Kim YM, Houpt TA, Kim DG. Adrenalectomy abolishes fasting-induced down-regulation of NADPH-diaphorase in the rat paraventricular nucleus. Yonsei Med J. 2004. 45:123–128.
5. Jahng JW, Lee JY, Yoo SB, Kim YM, Ryu V, Kang DW, Lee JH. Refeeding-induced expression of neuronal nitric oxide synthase in the rat paraventricular nucleus. Brain Res. 2005. 1048:185–192.
6. van Haasteren GA, Linkels E, van Toor H, Klootwijk W, Kaptein E, de Jong FH, Reymond MJ, Visser TJ, de Greef WJ. Effects of long-term food reduction on the hypothalamus-pituitary-thyroid axis in male and female rats. J Endocrinol. 1996. 150:169–178.
7. Yoshihara T, Honma S, Katsuno Y, Honma K. Dissociation of paraventricular NPY release and plasma corticosterone levels in rats under food deprivation. Am J Physiol. 1996. 271:E239–E245.
8. Lee JY, Kang DW, Kim DG, Jahng JW. Fasting-induced down-regulation of NADPH-diaphorase in the magnocellular PVN of rats. Yonsei Med J. 2004. 45:917–922.
9. Jeong Y, Won J, Kim C, Yim J. 5'-Flanking sequence and promoter activity of the rabbit neuronal nitric oxide synthase (nNOS) gene. Mol Cells. 2000. 10:566–574.
10. Rife TK, Xie J, Redman C, Young AP. The 5'2 promoter of the neuronal nitric oxide synthase dual promoter complex mediates inducibility by nerve growth factor. Brain Res Mol Brain Res. 2000. 75:225–236.
11. Sasaki M, Gonzalez-Zulueta M, Huang H, Herring WJ, Ahn S, Ginty DD, Dawson VL, Dawson TM. Dynamic regulation of neuronal NO synthase transcription by calcium influx through a CREB family transcription factor-dependent mechanism. Proc Natl Acad Sci USA. 2000. 97:8617–8622.
12. Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989. 59:675–680.
13. Son GH, Geum D, Jung H, Kim K. Glucocorticoid inhibits growth factor-induced differentiation of hippocampal progenitor HiB5 cells. J Neurochem. 2001. 79:1013–1021.
14. Lee JY, Lee JH, Kim DG, Jahng JW. Dexamethasone blocks the refeeding-induced phosphorylation of cAMP response element-binding protein in the rat hypothalamus. Neurosci Lett. 2003. 344:107–111.
15. Timofeeva E, Picard F, Duclos M, Deshaies Y, Richard D. Neuronal activation and corticotropin-releasing hormone expression in the brain of obese (fa/fa) and lean (fa/?) Zucker rats in response to refeeding. Eur J Neurosci. 2002. 15:1013–1029.
16. Sheriff S, Chance WT, Iqbal S, Rizvi TA, Xiao C, Kasckow JW, Balasubramaniam A. Hypothalamic administration of cAMP agonist/PKA activator inhibits both schedule feeding and NPY-induced feeding in rats. Peptides. 2003. 24:245–254.
17. Jahng JW, Spencer CM, Choi SH, Kim DG, Houpt TA. Nitric oxide is involved in lithium-induced immediate early gene expressions in the adrenal medulla. Eur J Pharmacol. 2004. 489:111–116.
18. Spencer CM, Jahng JW, Ryu V, Houpt TA. Lithium-induced gene expression of inducible cyclic adenosine monophosphate early repressor in the rat adrenal gland. J Neurosci Res. 2005. 82:273–282.
19. Lee JH, Cha MJ, Yoo SB, Moon YW, Noh SJ, Jahng JW. Leptin blocks the fasting-induced increase of pERK1/2 in the paraventricular nucleus of rats. Regul Pept. 2010. 162:122–128.
20. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 1986. New York: Academic Press.
21. Jahng JW, Houpt TA, Kim SJ, Joh TH, Son JH. Neuropeptide Y mRNA and serotonin innervation in the arcuate nucleus of anorexia mutant mice. Brain Res. 1998. 790:67–73.
22. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996. 382:250–252.
23. Boissel JP, Bros M, Schröck A, Gödtel-Armbrust U, Förstermann U. Cyclic AMP-mediated upregulation of the expression of neuronal NO synthase in human A673 neuroepithelioma cells results in a decrease in the level of bioactive NO production: analysis of the signaling mechanisms that are involved. Biochemistry. 2004. 43:7197–7206.
24. Eberhardt W, Engels C, Müller R, Pfeilschifter J. Mechanisms of dexamethasone-mediated inhibition of cAMP-induced tPA expression in rat mesangial cells. Kidney Int. 2002. 62:809–821.
25. Dostmann WR, Taylor SS, Genieser HG, Jastorff B, Døskeland SO, Ogreid D. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinases I and II with analogs of adenosine 3',5'-cyclic phosphorothioates. J Biol Chem. 1990. 265:10484–10491.
26. Wu G, Meininger CJ. Regulation of nitric oxide synthesis by dietary factors. Annu Rev Nutr. 2002. 22:61–86.
27. Park SH, Lee YJ, Lim MJ, Kim EJ, Lee JH, Han HJ. High glucose inhibits fructose uptake in renal proximal tubule cells: involvement of cAMP, PLC/PKC, p44/42 MAPK, and cPLA2. J Cell Physiol. 2004. 200:407–416.
28. Santangelo GM. Glucose signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2006. 70:253–282.
29. Otsubo H, Kondoh T, Shibata M, Torii K, Ueta Y. Induction of Fos expression in the rat forebrain after intragastric administration of monosodium L-glutamate, glucose and NaCl. Neuroscience. 2011. 196:97–103.
30. Bredt DS, Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992. 8:3–11.
31. Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995. 57:683–706.
32. Yun HY, Dawson VL, Dawson TM. Neurobiology of nitric oxide. Crit Rev Neurobiol. 1996. 10:291–316.
33. Boissel JP, Schwarz PM, Förstermann U. Neuronal-type NO synthase: transcript diversity and expressional regulation. Nitric Oxide. 1998. 2:337–349.
34. Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994. 14:5147–5159.
35. Förstermann U, Boissel JP, Kleinert H. Expressional control of the 'constitutive' isoforms of nitric oxide synthase (NOS I and NOS III). FASEB J. 1998. 12:773–790.
36. Casto RM, VanNess JM, Overton JM. Effects of central leptin administration on blood pressure in normotensive rats. Neurosci Lett. 1998. 246:29–32.
37. Krukoff TL. Central actions of nitric oxide in regulation of autonomic functions. Brain Res Brain Res Rev. 1999. 30:52–65.
38. Stern JE, Li Y, Zhang W. Nitric oxide: a local signalling molecule controlling the activity of pre-autonomic neurones in the paraventricular nucleus of the hypothalamus. Acta Physiol Scand. 2003. 177:37–42.
39. Kadowaki K, Kishimoto J, Leng G, Emson PC. Up-regulation of nitric oxide synthase (NOS) gene expression together with NOS activity in the rat hypothalamo-hypophysial system after chronic salt loading: evidence of a neuromodulatory role of nitric oxide in arginine vasopressin and oxytocin secretion. Endocrinology. 1994. 134:1011–1017.
40. Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983. 6:269–324.
41. Burlet AJ, Jhanwar-Uniyal M, Chapleur-Chateau M, Burlet CR, Leibowitz SF. Effect of food deprivation and refeeding on the concentration of vasopressin and oxytocin in discrete hypothalamic sites. Pharmacol Biochem Behav. 1992. 43:897–905.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download