Abstract
The hypothalamic-pituitary-adrenal (HPA) axis is the primary endocrine system to respond to stress. The HPA axis may be affected by increased level of corticotrophin-releasing factors under chronic stress and by chronic administration of monosodium glutamate (MSG). The purpose of this study was to investigate whether chronic MSG administration aggravates chronic variable stress (CVS)-induced behavioral and hormonal changes. Twenty-four adult male Sprague-Dawley rats, weighing 200∼220 g, were divided into 4 groups as follows: water administration (CON), MSG (3 g/kg) administration (MSG), CVS, and CVS with MSG (3 g/kg) administration (CVS+MSG). In addition, for the purpose of comparing the effect on plasma corticosterone levels between chronic stress and daily care or acute stress, 2 groups were added at the end of the experiment; the 2 new groups were as follows: naïve mice (n=7) and mice exposed to restraint stress for 2 h just before decapitation (A-Str, n=7). In an open field test performed after the experiment, the CVS+MSG group significant decrease in activity. The increase in relative adrenal weights in the CVS and CVS+MSG group was significantly greater than those in the CON and/or MSG groups. In spite of the increase in the relative adrenal weight, there was a significant decrease in the plasma corticosterone levels in the CVS+MSG group as compared to all other groups, except the naïve group. These results suggest that impaired HPA axis function as well as the decrease in the behavioral activity in adult rats can be induced by chronic MSG administration under CVS rather than CVS alone.
References
1. Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol Behav. 2007; 90:29–35.

2. Mathew SJ, Coplan JD, Schoepp DD, Smith EL, Rosenblum LA, Gorman JM. Glutamate-hypothalamic-pituitary-adrenal axis interactions: implications for mood and anxiety disorders. CNS Spectr. 2001; 6:555–556.

3. Zelena D, Mergl Z, Makara GB. Glutamate agonists activate the hypothalamic-pituitary-adrenal axis through hypothalamic paraventricular nucleus but not through vasopressinergic neurons. Brain Res. 2005; 1031:185–193.
4. Walker R, Lupien JR. The safety evaluation of monosodium glutamate. J Nutr. 2000; 130 Suppl. 4:1049S–1052S.

5. Lucas DR, Newhouse JP. The toxic effect of MSG on the inner layers of the retina. AMA Arch Ophthalmol. 1957; 58:193–201.
6. Larsen PJ, Mikkelsen JD, Jessop D, Lightman SL, Chowdrey HS. Neonatal monosodium glutamate treatment alters both the activity and the sensitivity of the rat hypothalamo-pituitary-adrenocortical axis. J Endocrinol. 1994; 141:497–503.

7. Beyreuther K, Biesalski HK, Fernstrom JD, Grimm P, Hammes WP, Heinemann U, Kempski O, Stehle P, Steinhart H, Walker R. Consensus meeting: monosodium glutamate – an update. Eur J Clin Nutr. 2007; 61:304–313.

8. Magariños AM, Estivariz F, Morado MI, De Nicola AF. Regulation of the central nervous system-pituitary-adrenal axis in rats after neonatal treatment with monosodium glutamate. Neuroendocrinology. 1988; 48:105–111.

9. Tirassa P, Lundeberg T, Stenfors C, Bracci-Laudiero L, Theodorsson E, Aloe L. Monosodium glutamate increases NGF and NPY concentrations in rat hypothalamus and pituitary. Neuroreport. 1995; 6:2450–2452.

10. Monno A, Vezzani A, Bastone A, Salmona M, Garattini S. Extracellular glutamate levels in the hypothalamus and hippocampus of rats after acute or chronic oral intake of monosodium glutamate. Neurosci Lett. 1995; 193:45–48.

11. Bogdanov MB, Tjurmina OA, Wurtman RJ. Consumption of a high dietary dose of monosodium glutamate fails to affect extracellular glutamate levels in the hypothalamic arcuate nucleus of adult rats. Brain Res. 1996; 736:76–81.

12. Russell BL. Excitotoxins, The Taste that kills. 1st ed.Santa Fe: New Mexico, Health Press NA Inc;1997. p. 33–57.
13. Kim YS, Lee MY, Choi CS, Sohn YW, Park BR, Choi MG, Nah YH, Choi SC. The effect of chronic variable stress on bowel habit and adrenal function in rats. J Gastroenterol Hepatol. 2008; 23:1840–1846.

14. Kennett GA, Chaouloff F, Marcou M, Curzon G. Female rats are more vulnerable than males in an animal model of depression: the possible role of serotonin. Brain Res. 1986; 382:416–421.

15. Krahn DD, Gosnell BA, Majchrzak MJ. The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biol Psychiatry. 1990; 27:1094–1102.

16. Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui D, Tabira T. Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology. 2001; 26:443–459.

17. Martí O, Martí J, Armario A. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav. 1994; 55:747–753.

18. Sekino A, Ohata H, Mano-Otagiri A, Arai K, Shibasaki T. Both corticotropin-releasing factor receptor type 1 and type 2 are involved in stress-induced inhibition of food intake in rats. Psychopharmacology (Berl). 2004; 176:30–38.

19. Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999; 46:1167–1180.

20. Krahn DD, Gosnell BA, Grace M, Levine AS. CRF antagonist partially reverses CRF- and stress-induced effects on feeding. Brain Res Bull. 1986; 17:285–289.

21. Diniz YS, Faine LA, Galhardi CM, Rodrigues HG, Ebaid GX, Burneiko RC, Cicogna AC, Novelli EL. Monosodium glutamate in standard and high-fiber diets: metabolic syndrome and oxidative stress in rats. Nutrition. 2005; 21:749–755.

22. Swiergiel AH, Leskov IL, Dunn AJ. Effects of chronic and acute stressors and CRF on depression-like behavior in mice. Behav Brain Res. 2008; 186:32–40.

23. Hlinák Z, Gandalovicová D, Krejcí I. Behavioral deficits in adult rats treated neonatally with glutamate. Neurotoxicol Teratol. 2005; 27:465–473.
24. Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav. 2003; 78:703–710.

25. D'Aquila PS, Brain P, Willner P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav. 1994; 56:861–867.
26. Kopp C, Vogel E, Rettori MC, Delagrange P, Misslin R. The effects of melatonin on the behavioural disturbances induced by chronic mild stress in C3H/He mice. Behav Pharmacol. 1999; 10:73–83.

27. Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand JP, Soubrié P. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-m ethylphenyl)ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther. 2002; 301:333–345.
28. Buelke-Sam J, Kimmel CA, Nelson CJ, Sullivan PA. Sex and strain differences in the developmental activity profile of rats prenatally exposed to sodium salicylate. Neurobehav Toxicol Teratol. 1984; 6:171–175.
29. Kim DG, Lee S, Lim JS. Neonatal footshock stress alters adult behavior and hippocampal corticosteroid receptors. Neuroreport. 1999; 10:2551–2556.

30. Huether G. The central adaptation syndrome: psychosocial stress as a trigger for adaptive modifications of brain structure and brain function. Prog Neurobiol. 1996; 48:569–612.

31. Luo DD, An SC, Zhang X. Involvement of hippocampal serotonin and neuropeptide Y in depression induced by chronic unpredicted mild stress. Brain Res Bull. 2008; 77:8–12.

32. Wang D, An SC, Zhang X. Prevention of chronic stress-induced depression-like behavior by inducible nitric oxide inhibitor. Neurosci Lett. 2008; 433:59–64.

33. Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res. 2005; 161:45–59.

34. Kim KS, Han PL. Optimization of chronic stress paradigms using anxiety- and depression-like behavioral parameters. J Neurosci Res. 2006; 83:497–507.

35. D'Aquila PS, Peana AT, Carboni V, Serra G. Exploratory behaviour and grooming after repeated restraint and chronic mild stress: effect of desipramine. Eur J Pharmacol. 2000; 399:43–47.
36. Holsboer F. The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res. 1999; 33:181–214.

37. Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999; 160:1–12.

38. Makara GB, Stark E. Effect of intraventricular glutamate on ACTH release. Neuroendocrinology. 1975; 18:213–216.

39. Chautard T, Boudouresque F, Guillaume V, Oliver C. Effect of excitatory amino acid on the hypothalamo-pituitary-adrenal axis in the rat during the stress-hyporesponsive period. Neuroendocrinology. 1993; 57:70–78.

40. Jezová D, Tokarev D, Rusnák M. Endogenous excitatory amino acids are involved in stress-induced adrenocorticotropin and catecholamine release. Neuroendocrinology. 1995; 62:326–332.
41. Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000; 25:1–35.

42. Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003; 160:1554–1565.

43. Pignatelli D, Maia M, Castro AR, da Conceição Magalhães M, Vivier J, Defaye G. Chronic stress effects on the rat adrenal cortex. Endocr Res. 2000; 26:537–544.

44. Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005; 11:963–972.

Fig. 1.
Time-dependent changes of body weight in each group for 7 weeks. ∗Denotes significant differences among groups based on the one-way ANOVA and Bonferroni test (∗p<0.05). There are significant differences in the body weight between CON and other groups; an especially remarkable decrease could be seen in the CVS +MSG group. CON, control; MSG, monosodium glutamate; CVS, chronic variable stress.
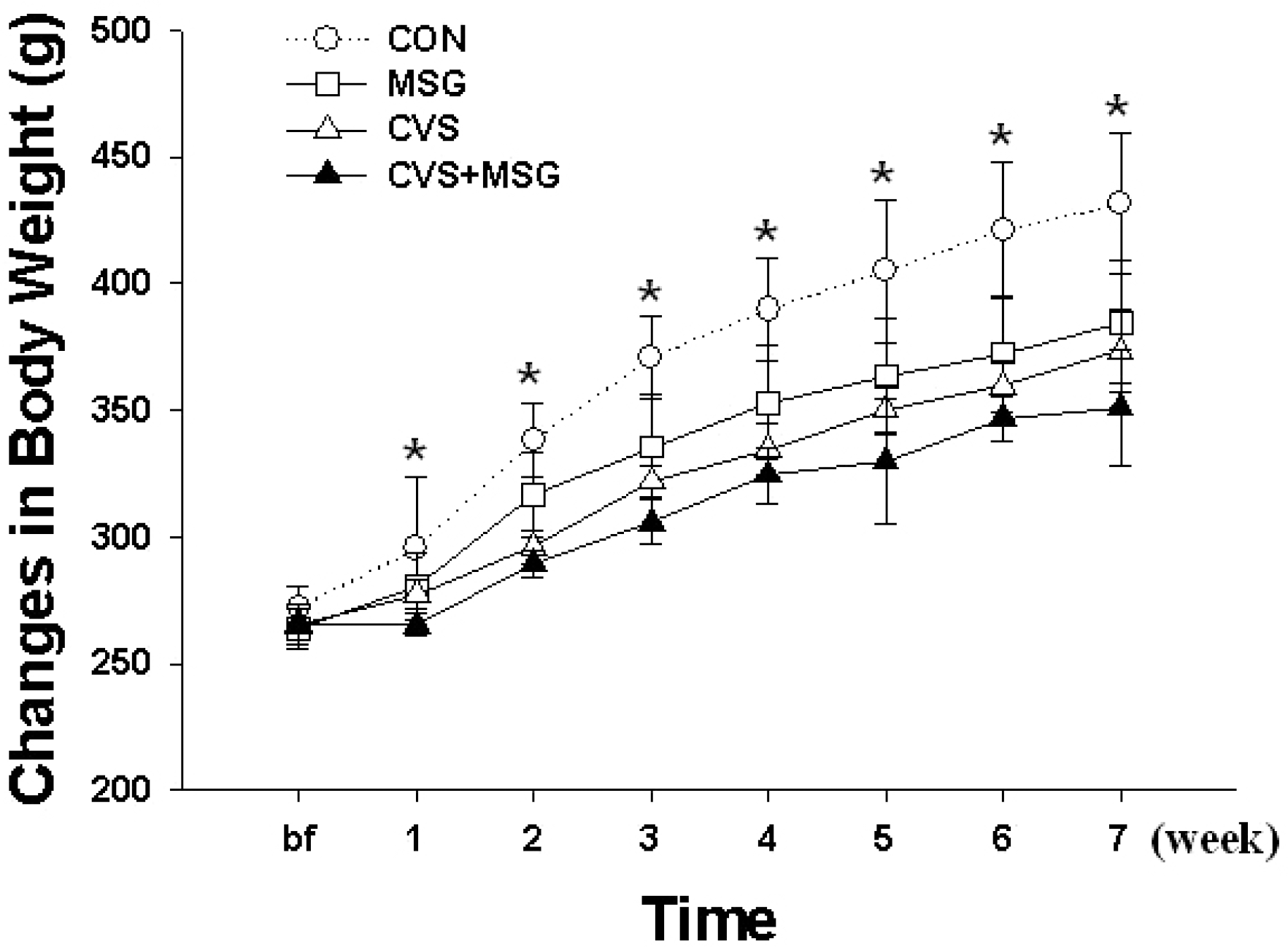
Fig. 2.
Time-dependent changes of food consumption in each group for 7 weeks. ∗Denotes significant differences among groups based on the one-way ANOVA and Bonferroni test (∗p<0.05). There are significant differences in the daily food intake between the control (CON) and other groups. The difference in food intake between the groups gradually decreased during the course of the experiment, except in the CON group. CON, control; MSG, monosodium glutamate; CVS, chronic variable stress.
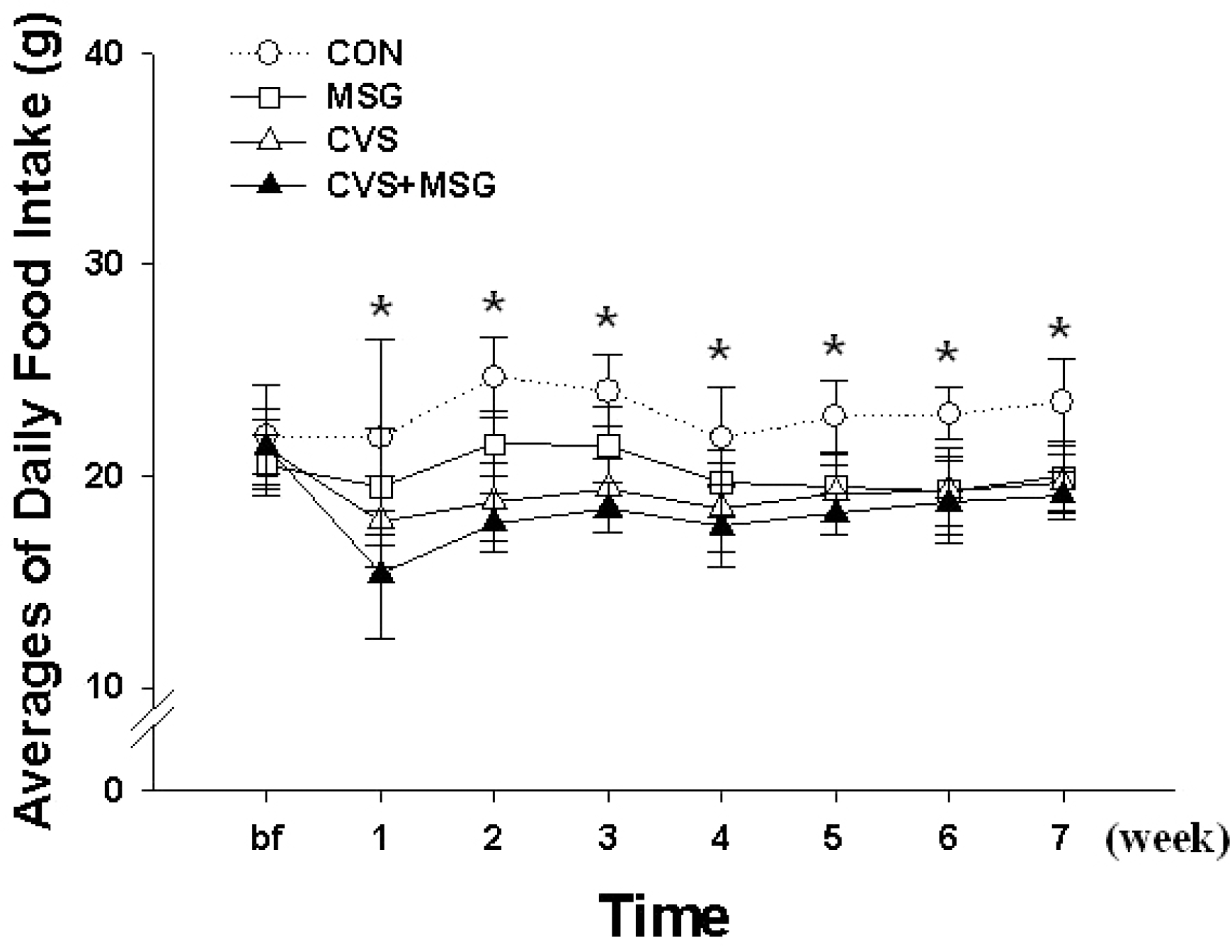
Fig. 3.
Behavioral response before the stress experiment in the open field test. The stereotypic and exploring activity gradually decreased as time passed in all groups. There is no significant difference in all parameters between the groups. CON, control; MSG, monosodium glutamate; CVS, chronic variable stress.
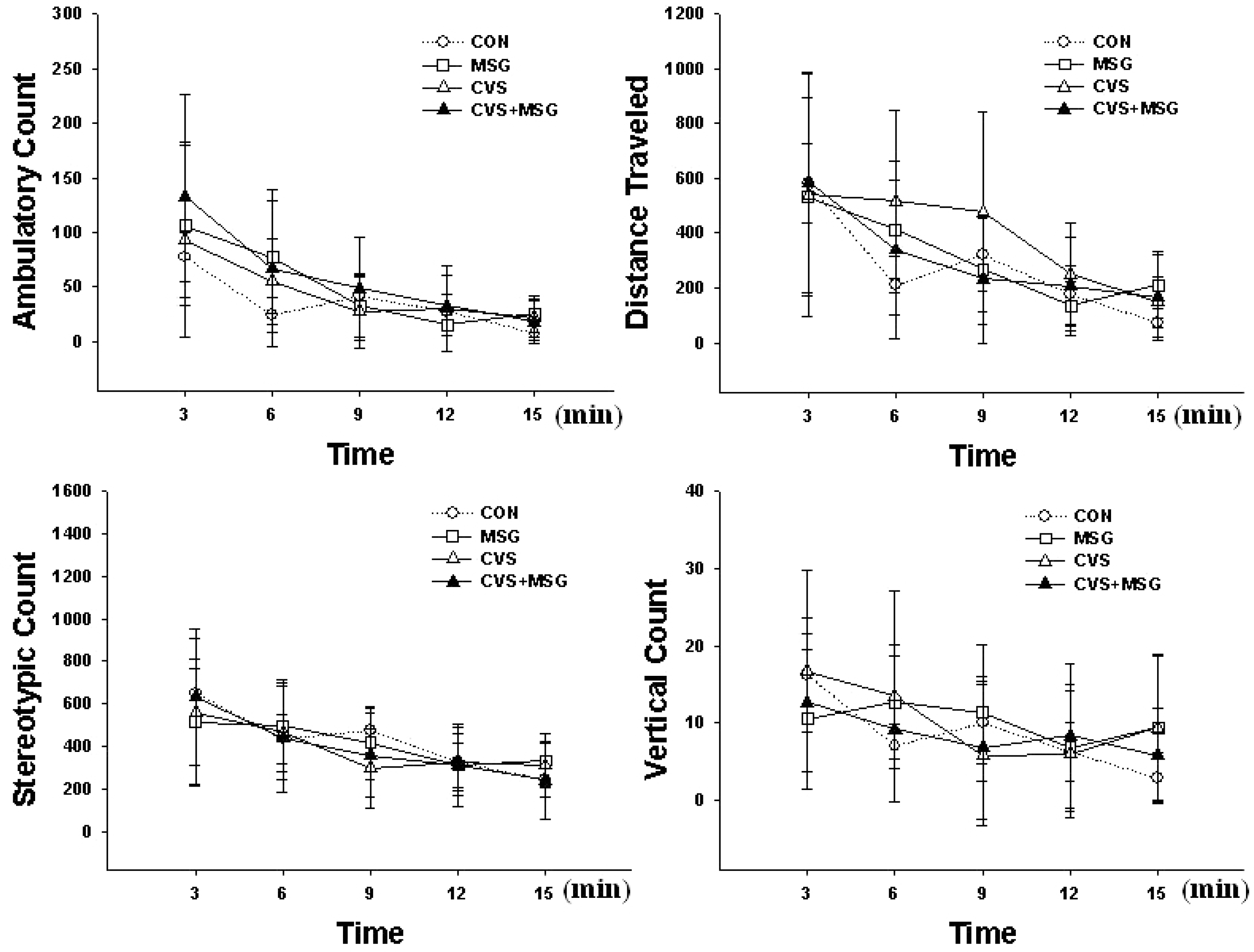
Fig. 4.
Behavioral response after the stress experiment in the open field test. There are significant differences in all parameters between the groups, especially the rats in the CVS+MSG group showed a remarkable decrease in their activity in the stereotypic count measurement. ∗Denotes significant differences among the groups based on the one-way ANOVA and Bonferroni test (∗p <0.05). CON, control; MSG, monosodium glutamate; CVS, chronic variable stress.
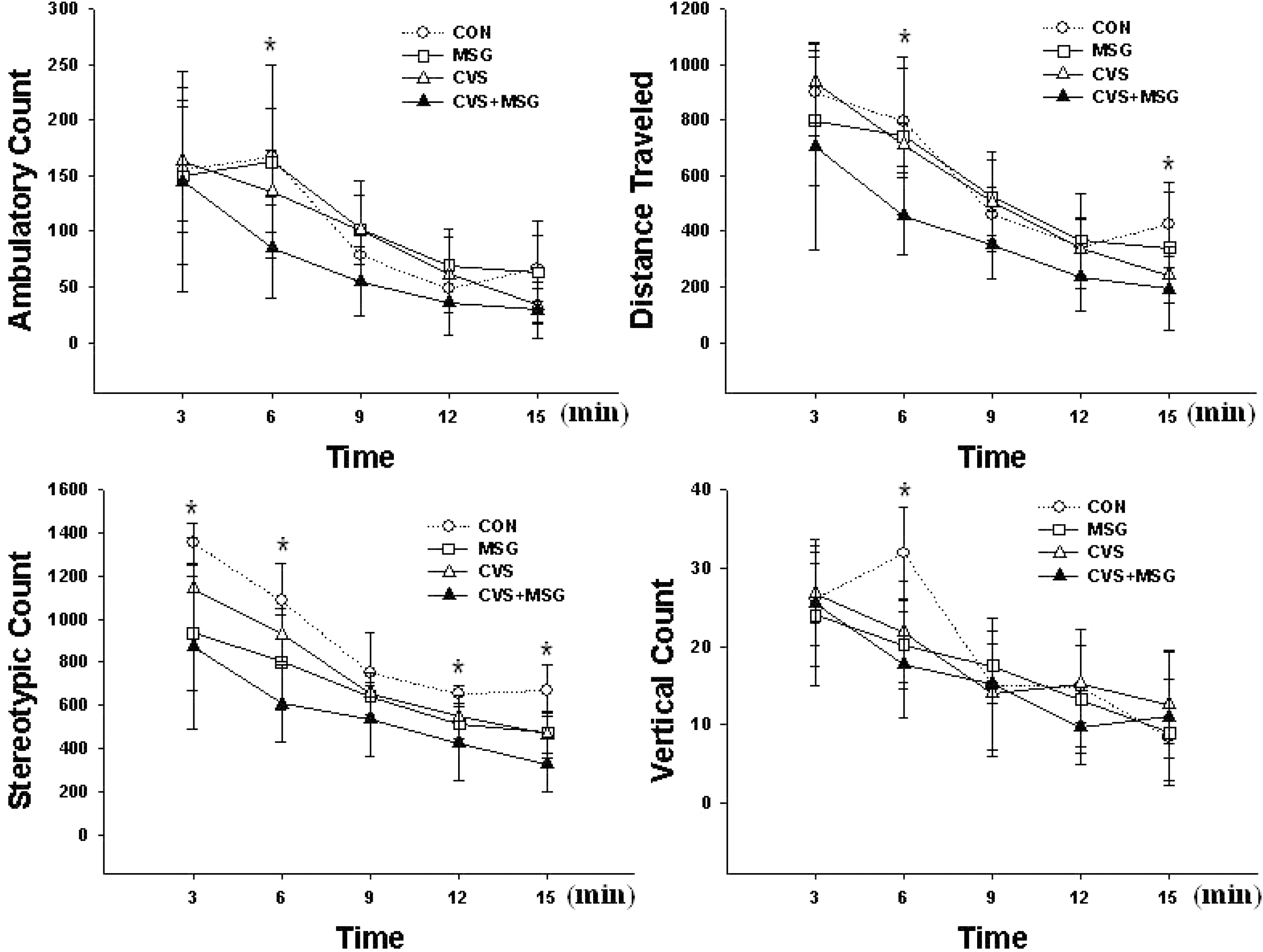
Fig. 5.
Relative adrenal weight following administration of MSG for 7 weeks. Statistical analysis was performed by the one-way ANOVA and Bonferroni test. The relative adrenal weight to body weight ratio was significantly higher in the CVS and CVS + MSG group than in the CON group. In addition, although there was no significant difference in the relative adrenal weight between the MSG and CVS group, the relative adrenal weight in the CVS + MSG group (0.75±0.09) was significantly higher than in the MSG group. ∗Denotes significant differences between CON and other groups (∗p <0.05). †Denotes significant difference between the stress and CVS + MSG group (†p<0.05). #Denotes significant difference between the MSG and CVS + MSG group (#p<0.05). CON, control; MSG, monosodium glutamate; CVS, chronic variable stress; A-Str, restraint stress for 2 hours just before decapitation; Wt, weight.
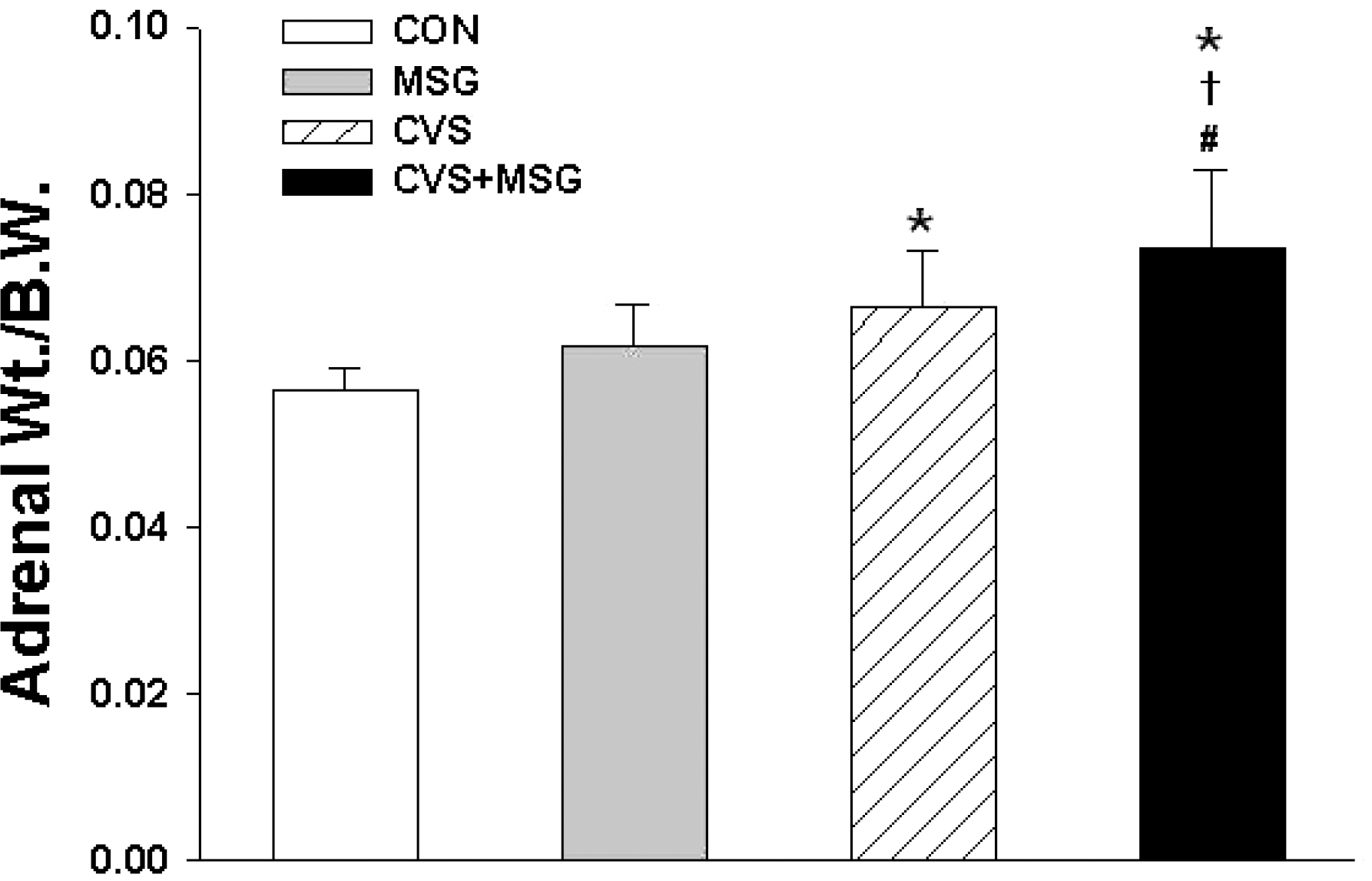
Fig. 6.
Plasma corticosterone levels in each group following 7 weeks experiment. Statistical analysis was performed by the one-way ANOVA and Bonferroni test. There was a significant difference in the plasma corticosterone levels between the naïve group and all other groups, including the CON group. $Denotes significant differences between the naïve and other groups ($p<0.05). ∗Denotes significant difference between CON and other groups (∗p <0.05). †Denotes significant difference between CVS + MSG and A-Str group (†p<0.05). CON, control; MSG, monosodium glutamate; CVS, chronic variable stress; A-Str, restraint stress for 2 hours just before decapitation.
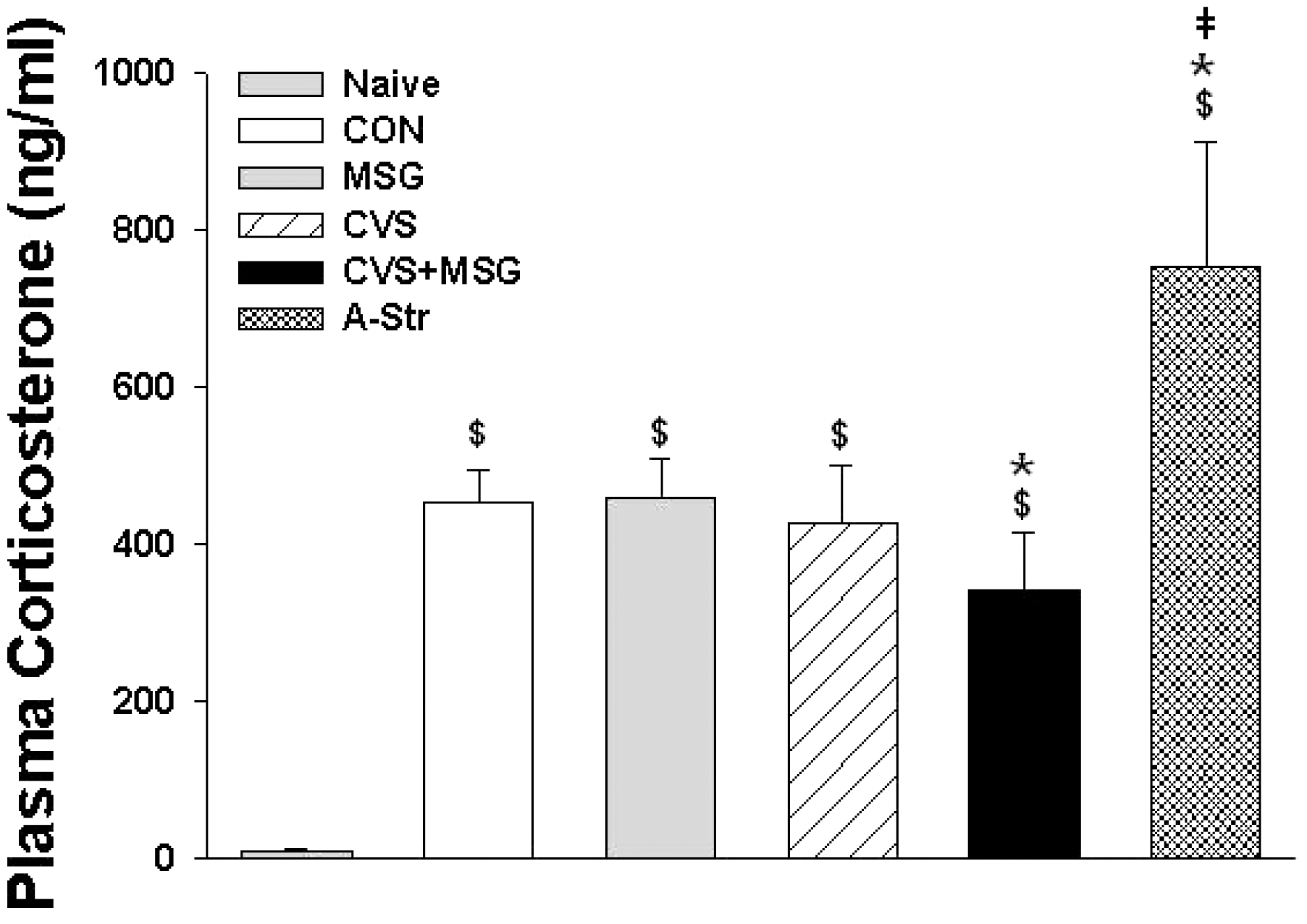
Table 1.
Chronic variable stress protocol




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download