Abstract
Purpose
There are fewer patients with gastroesophageal reflux disease (GERD) in Korea compared with Western countries. The incidence of GERD has increased in recent years however, concerning many physicians. Here, we report our early experiences of using a recently introduced method of laparoscopic antireflux surgery for the treatment of GERD in Korean patients.
Methods
Fifteen patients with GERD were treated using antireflux surgery between May 2009 and February 2012 at the University of Ulsan College of Medicine and Asan Medical Center. Laparoscopic Nissen fundoplication with 360° wrapping was performed on all patients.
Results
Eleven male and four female patients were evaluated and treated with an average age of 58.1 ± 14.1 years. The average surgical time was 118.9 ± 45.1 minutes, and no complications presented during surgery. After surgery, the reflux symptoms of each patient were resolved; only two patients developed transient dysphagia, which resolved within one month. One patient developed a 6-cm hiatal hernia that had to be repaired and reinforced using mesh.
Conclusion
The use of laparoscopic surgery for the treatment of GERD is safe and feasible. It is also an efficacious method for controlling the symptoms of GERD in Korean patients. However, the use of this surgery still needs to be standardized (e.g., type of surgery, bougienage size, wrap length) and the long-term outcomes need to be evaluated.
As gastroesophageal reflux disease (GERD) continues to become one of the most common gastric diseases in Western countries, thereby reducing quality of life and causing a variety of complications, it increasingly receives more public attention as well as concern among physicians. GERD is defined as a constellation of symptoms and side effects that damage the gastric mucous membranes and esophagus while the contents are refluxed [1]. Recently, laryngitis, coughing, asthma, and dental erosion (i.e., extraesophageal symptoms), along with esophageal symptoms and endoscopic indications, were proposed as the major symptoms for the diagnosis of GERD. In addition, in order to ensure an accurate diagnosis, patient-oriented approaches that utilize segmented classification techniques, which depend on the diagnosis of each symptom, are required [1].
According to a population-based study, GERD afflicts approximately 10% to 20% of North Americans and Europeans when defined as experiencing >1 episode of heartburn or acid reflux per week; in Asia, the prevalence is <10%. The annual incidence of GERD in Western countries is 5 persons per 1,000 persons, and its incidence is associated with various risk factors such as medical family history, obesity, and concomitant disease such as respiratory system disease [2].
The prevalence of GERD is relatively low in Korea but has rapidly increased in recent years as dietary habits have become more westernized. The prevalence of GERD in Korea demonstrates significant differences depending on the study, but it is known to afflict 5% to 17% of adults [3,4].
The use of proton pump inhibitors is recognized as the optimal therapeutic method for treating esophagitis, demonstrating improvements in GERD symptoms of up to 85%; however, symptoms recur in 80% of patients when medication is stopped or, in some cases, when the patient is undermedicated, resulting in persistent reflux.
After Nissen reported improvements in reflux symptoms in 1955 (quoted from [5]) following the implementation of fundoplication, which uses the gastric fundus to reinforce the lower esophageal sphincter, concerns over the use antireflux surgery have become more common. In particular, the laparoscopic surgery method introduced by Dallemagne et al. [6] in 1991 has been rapidly propagated, and now the use of standardized and laparoscopic fundoplication contribute to the treatment of GERD. However, due to the uncertainty of the surgical indications, the insufficient number of surgeons who have the appropriate amount of experience performing laparoscopic procedures, and the lack of data regarding the use of antireflux surgery to treat patients, laparoscopic fundoplication has not been universally or commonly implemented in Korea [7].
Taking this information into account, we analyzed in our current study the clinical results of 15 patients who were treated using antireflux surgery (Nissen fundoplication) at the Department of Surgery of Asan Medical Center (University of Ulsan College of Medicine, Seoul, Korea) in order to collect reference data on the use of this treatment in Korea.
Fifteen patients were identified who underwent laparoscopic Nissen fundoplication due to GERD at Asan Medical Center, and a retrospective analysis was performed on the medical records of these patients. Symptom improvement was noted on the follow-up examinations of 45 outpatients who were administered proton pump inhibitors at the Department of Gastroenterology, Asan Medical Center. Of these patients, 15 patients were referred to the Department of Surgery to undergo antireflux surgery for the treatment of GERD. Indications for surgery that were requested by the Department of Internal Medicine included the following: 1) no reaction despite treatment with proton pump inhibitors for >6 months (e.g., the continuation of nonacid reflux, the presence of a large hiatal hernia, partial acid sensitivity); 2) an inability to discontinue the use of proton pump inhibitors because these medications were required to maintain therapeutic effects; and 3) the continuation of serious extraesophageal symptoms such as coughing or asthma [8].
Endoscopic examination of the upper gastrointestinal (GI) tract, a 24-hour esophageal pH measurement, esophageal manometry, and barium esophagography were performed as preoperative evaluations and, in the case of laryngopharyngeal symptoms, evaluation at the ENT (ear, nose, and throat) department was performed in parallel. Examinations were omitted due to the presence of a large hiatal hernia or patient refusal.
Esophagitis was classified according to the Los Angeles method using upper GI endoscopy, and esophageal motility disorder was diagnosed using esophageal manometry. In addition, the DeMeester score was determined by measuring the 24-hour esophageal pH.
Laparoscopic Nissen fundoplication, which forms an acute His angle by creating an antireflux valve system, wrapping the gastroesophageal junction, and directing the gastric fundus to the esophageal anterior, was performed on every patient. First, after placing the patient in the supine position, the patient was tilted into the reverse Trendelenburg stance at 20°. Five trocars were used, and their locations are shown in Fig. 1. Trocars (12 mm) for the camera were inserted into a site that was 2 cm to the left of the upper part of the umbilicus by 3 cm and as working port. A trocar (5 mm) was also inserted into the right upper abdomen, and another (12 mm) into the left upper abdomen. A 5 mm port in the epigastrium was used for liver retraction. Another 5 mm port for the assistant was placed on the left posterior axillary line under the working trocar. After exfoliating the gastrophrenic ligament using various laparoscopic devices, the area around the lower abdominal esophagus was sufficiently exfoliated without damaging the vagus nerve. The abdominal esophagus was pulled to the left side of the patient using U-tape. Using this procedure, a 5 cm section of the abdominal esophagus was secured. The diaphragm was widened enough to insert the bougie, and the gap between the right and left crura was narrowed using 1 to 2 nonabsorbable sutures. At this point of the procedure, postoperative dysphasia can be prevented only when the provided space is wide enough to allow the soft passage of just one piece of Kelly clamp forceps. The gastric fundus was made freely movable by exfoliating and ligating the short gastric vessels. A temporary wrap was made by pulling the most distal portion of the gastric fundus and inserting forceps into the anterior abdominal esophagus. A 40 to 60 Fr bougie was inserted at this time, and the gastric fundus was made fully movable so that the wrap would not return to its original position. Tension in the wrap was confirmed by performing the "shoeshine maneuver".
In order to make the wrap both short and loose, an approximately 2 cm gastric fundus wrap was made and suturing was usually performed using 2 to 3 nonabsorbable sutures. The first suture prevented movement of the wrap within the muscular layer of the completely fibrotic esophageal wall. In addition, by inserting one laparoscopic forcep under the wrap, the forcep could be conveniently moved.
Age, sex, obesity, symptoms, symptom duration, surgical time, complications, and the results of all follow-up examinations were evaluated in the target patients. Postoperative progress, dysphagia status, and the medication status of any proton pump inhibitors were confirmed and evaluated using the Visick scoring system, 4 stage progress was observed. Acute complications due to surgery included one patient who required another operation, one patient who was also treated using endoscopy or radiology, and one patient whose discharge was delayed by >2 days. The dysphasic symptoms of these patients are described in a separate study.
IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA) was used to perform all of the statistical analyses. Statistical significance was defined as a P-value < 0.05. Data are shown as the average ± standard deviation, and the data were evaluated using descriptive statistics and frequency analysis.
We have performed laparoscopic Nissen fundoplication on 15 adult GERD patients since 2009. At the time of their operations, the average age of these patients was 58.1 ± 14.1 years, the male:female gender ratio was 11:4, and the mean body mass index was 24.9 ± 3.1. Major symptoms included heartburn (12 patients), regurgitation (10 patients), dysphasia (5 patients), respiratory organ symptoms (4 patients), abdominal pain (2 patients), chest pain (2 patients), nausea and vomiting (2 patients), and excessive belching (1 patient) (Table 1). Typical symptoms were observed in all preoperative patients, and concomitant atypical symptoms were noted in 20% of patients. In addition, the average duration of symptoms was 5.3 ± 3.7 years.
Upper GI endoscopy, 24-hour esophageal pH measurement, esophageal manometry, and barium esophagography were performed as preoperative evaluations. All 15 patients demonstrated normal peristaltic esophageal motility. In terms of their DeMeester scores, four patients received normal scores and seven patients received high scores, demonstrating a mean value of 25.3 ± 34.2 points. Four patients were not evaluated using DeMeester scoring. Regarding the severity of esophagitis based on the Los Angeles classification system, 40% (6 patients) were classified as grade M, 20% (3 patients) as grade A, 26% (4 patients) as grade B, 7% (1 patient) as grade C, and 7% (1 patient) with a normal esophagus (Table 2).
Hiatal hernia was observed in 11 patients using endoscopy, reducible hernia with a length of 3-4 cm was observed in 10 patients, and nonreducible hiatal hernia with a length >6 cm was observed in one patient. Previous abdominal surgery was confirmed in four patients, including two patients who had undergone a cholecystectomy, one patient who had undergone an appendectomy, and one patient who had undergone gynecological surgery.
In all 15 patients in our study cohort, laparoscopic Nissen fundoplication was performed in order to wrap the gastric fundus by 360°. The average surgical time was 118.9 ± 45.1 minutes, and the average size of the bougie that was used was 45.5 ± 2.2 Fr. Bleeding was maintained at <50 mL. Conversion to laparotomy was not performed on any patient (Table 3).
Hiatal hernia was postoperatively observed in all four patients who were not initially diagnosed with preoperative hiatal hernia. Repair was not required in one of these patients. In the remaining three patients, hernias were repaired using 2-0 Prolene sutures at 2-3 sites. In the 11 patients with preoperative hiatal hernia, repair was performed by reinforcement with 15 cm × 15 cm dual mesh in one patient who had a 6-cm hiatal hernia. In the remaining 10 cases, hernia repair was performed using 2-0 Prolene sutures at 2-3 sites. In addition, cholecystectomy was performed at the same time on one patient who was diagnosed with concomitant chronic cholecystitis.
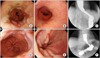
No surgical complications developed in any of the patients during their hospitalizations. In two cases, gastrointestinography using gastrografin was performed on the day after surgery and indicated transient dysphasia concomitant with slight stenosis, but these symptoms disappeared within one month. The average duration of hospitalization was 5 days (range, 3 to 8 days), and during the first 2 to 3 weeks after surgery all patients were advised to only ingest soft foods and fluids (e.g., thin rice gruel, porridge) and avoid solid foods (e.g., dried bread, steak). These patients were able to return to their normal activities within an average of three weeks. The average follow-up observation period was 16 ± 7 months, and in 12 patients the administration of proton pump inhibitors was not required because their symptoms had completely resolved. Proton pump inhibitors were administered to two patients at a half-dose because their symptoms remained, and one patient required the sustained administration of proton pump inhibitors due to the continuation of symptoms. Because the follow-up period was short and recurrence did not take place, follow-up examinations were not performed on 14 patients. Fig. 2 shows the results of the perioperative upper GI endoscopies and barium esophagographies that were used to evaluate the patients who did and did not demonstrate postoperative improvement.
Approximately 85% of GERD patients demonstrate improvement following treatment with drugs that inhibit the secretion of gastric acid (e.g., proton pump inhibitors, H2-receptor blockers), but if any issues with the mechanical antireflux defense system in the lower esophagus are not resolved then surgery is required to repair this problem. Since 1987, when Mouret announced the successful use of laparoscopic cholecystectomy, laparoscopic procedures have been successfully used to perform fundoplication. Following the long-term observation of 2,453 patients with GERD, Perdikis et al. [9] reported that the clinical results of laparoscopic surgery are the same as laparotomy, concluding that the method is safe and results in limited sequelae.
Currently, Nissen fundoplication, which forms a complete 360° wrap around the esophagus using the gastric fundus, is the most frequently performed surgery for the treatment of GERD. In addition to this method, Toupet fundoplication (which forms a 270° wrap around the esophageal anterior), Dor fundoplication (which forms a 150°-200° anterior wrap), and the Belsy Mark TV procedure (which forms a 270° anterior wrap through the chest) can also be performed. Because Nissen fundoplication severely increases the static pressure of the lower esophageal sphincter, resistance against esophageal regurgitation will typically increase. Therefore, symptoms such as dysphasia, excessive belching, and gas distension may present in some patients. To prevent these symptoms, "short and loosened" Nissen fundoplication using 2-cm short wraps is currently recommended. Lund et al. [10] insists that the results of partial Toupet fundoplication are outstandingly superior to the Nissen procedure because the lower esophageal sphincter can be relieved in patients with esophageal motility disorder. However, Horvath et al. [11] reported that 50% of patients who undergo partial fundoplication demonstrate evidence of reflux, and, noting this report, Limpert and Naunheim [12] emphasized that Nissen fundoplication is the best method if short gastric artery and vein division and suturing of the crural diaphragm can be performed using a 60-Fr bougie, even in patients with moderate esophageal motility disorder.
The recommended bougie is 54 to 60 Fr, but in our present study cohort the operations were feasible using bougies measuring 45.5 ± 2.2 Fr. Because all 15 patients in our current study demonstrated normal esophageal motility, Nissen fundoplication was performed in every case. In patients with weak esophageal motility, partial fundoplication, including Toupet fundoplication, may be required and should at least be considered. Intraoperative complications, such as bleeding, spleen damage, liver damage, and esophageal and gastric perforation may result from mistakes made during surgery, and postoperative complications, such as postoperative intestinal obstruction and urinary retention, may also develop. Pneumothorax and subcutaneous emphysema may present in 3% of patients, though wrap necrosis is rarely reported. Postoperative dysphasia may develop in 20% of the patients during the early stages, but most symptoms resolve within 3 months. However, esophageal dilatation may be required in some patients.
Postoperative complications are generally reported in approximately 7% of patients. Among the 15 patients reported here, intraoperative and perioperative complications did not present and conversion to laparotomy was not performed. In our present study, favorable results were found for 14 patients (93.3%), but the development of postoperative dysphasia still remains controversial. Upper GI series, gastric endoscopy, esophagography, and the gastric emptying test were performed on the two patients who complained of dysphasia, but indications of narrowed crural diaphragm sutures, loosened fundoplication, torsion, or long or narrow esophageal wraps were not observed. Brillantino et al. [13] and Kaul et al. [14] have proposed that pressure against the vagus nerve as the crural diaphragm is narrowed during hiatal hernia restoration is the cause of postoperative complications. In addition, we believe that one of our patients may have developed transient dysphasia due to hiatal hernia reduction.
Postoperative quality of life was exceptionally enhanced in the two patients in our cohort who demonstrated partial symptom improvement. In Japan, which is similar to Korea in terms of the incidence and treatment of GERD, 89.5% of postoperative patients demonstrate symptom improvement [15].
Barrett's esophagus is diagnosed in 5-15% of GERD patients. The natural course of Barrett's esophagus after antireflux surgery is controversial because severe symptoms or reflux often recur in these cases. According to various studies, low-grade dysplasia becomes nondysplastic in 44% of patients following antireflux surgery, and no cases of progression to esophageal adenocarcinoma have been observed. Therefore, some studies emphasize that because antireflux surgery affects the progress of Barrett's esophagus by preventing damage to the mucosal membrane of the esophagus by gastric acid, patients with GERD and Barrett's esophagus may be recommended for surgery. However, because it is difficult to conclude that antireflux surgery prevents progression to Barrett's esophagus, regular endoscopic examinations are required to monitor the postoperative incidence of metaplasia.
The results of our curret study also support the results reported by Hofstetter et al. [16] by demonstrating favorable results for Nissen fundoplication in the treatment of Barrett's esophagus. Although the number of patients reported here is limited and the duration of follow-up observations was short, our analysis shows that laparoscopic Nissen fundoplication is a very safe and valuable therapeutic method that effectively improves symptoms, treats esophagitis, and inhibits the secretion of gastric acid. If the concerns of gastroenterological surgeons continue to be addressed, the use of Nissen fundoplication for the treatment of GERD surgery should increase, and, therefore, it is expected that this method could become one of the most important therapeutic interventions for GERD.
In conclusion, laparoscopic Nissen fundoplication is not frequently performed in Asian countries, but is a safe and effective method for the treatment of GERD. However, because our current patients were not treated using identical internal treatments, our postoperative results were determined using subjective evaluations of patient improvement rather than the use of objective standards or other relevant data. Factors that may have affected the results of this study, such as a 2C19 polymorphism and genotype status, were not considered and our postoperative examinations and surgical indications were not standardized. Additional studies and consensus among experts will be required in the future to evaluate this treatment approach. In addition, a large study on the long-term effects of this surgery and its standardization (e.g., bougie size, wrap length, surgical method, fundoplication patterns) will be required.
Figures and Tables
Fig. 1
Trocar placement for laparoscopic fundoplication. Two 12 mm trocars and three 5 mm trocars are shown.
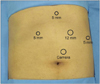
Fig. 2
(A-C) A 42-year-old female patient was administered 30 mg lansoprazole QD for 17 months, but was hospitalized due to the lack of improvement in dysphagia. (A) Preoperative upper gatrointestinal (GI) endoscopy. Semicircular erosions (occupying about 75% of the lumen) are noted on the lower esophagus, including multiple linear mucosal breaks. Focal upward projection of the Z-line with salmon-colored mucosal changes is also noted. (B) Fourteen months later, postoperative upper GI endoscopy was performed, demonstrating that the erosion of the Z-line was alleviated and reflux esophagitis was resolved. (C) Seven months later, postoperative barium esophagography was performed, demonstrating no evidence of extraluminal contrast leakage or significant contrast passage disturbances at the gastroesophageal junction. Proximal esophageal dilatation improved but still remained. Symptoms were alleviated after surgery, so the patient stopped taking lansoprazole. (D-F) A 67-year-old female patient was administered 40 mg esomeprazole QD for 8 months, but was hospitalized due to the lack of improvement in regurgitation. (D) Preoperative upper GI endoscopy. Reflux esophagitis is noted on the lower esophagus, including two 6 mm mucosal breaks. (E) After 28 months, postoperative upper GI endoscopy was performed, demonstrating reflux esophagitis with recurrent mucosal breaks and ulcerations. (F) After three months, a postoperative barium esophagography was performed, demonstrating mild luminal narrowing at the anastomotic site and no significant passage disturbances. Eight months after surgery, the patient is still being administered the same dose of esomeprazole for five months due to the lack of symptom improvement.
References
1. Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006. 101:1900–1920.
2. Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005. 54:710–717.
3. Ho KY, Cheung TK, Wong BC. Gastroesophageal reflux disease in Asian countries: disorder of nature or nurture? J Gastroenterol Hepatol. 2006. 21:1362–1365.
4. Jeon SG, Sohn CI, Kim JE, Park KH, Whang IS, Kim EJ, et al. Prevalence of gastroesophageal reflux in routine check-up subjects. Korean J Med. 2000. 58:145–151.
5. Christian DJ, Buyske J. Current status of antireflux surgery. Surg Clin North Am. 2005. 85:931–947.
6. Dallemagne B, Weerts JM, Jehaes C, Markiewicz S, Lombard R. Laparoscopic Nissen fundoplication: preliminary report. Surg Laparosc Endosc. 1991. 1:138–143.
7. Katada N. Review article: surgical and endoscopic therapy of gastro-oesophageal reflux disease in Japan. Aliment Pharmacol Ther. 2004. 20:Suppl 8. 28–31.
8. Stefanidis D, Hope WW, Kohn GP, Reardon PR, Richardson WS, Fanelli RD, et al. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc. 2010. 24:2647–2669.
9. Perdikis G, Hinder RA, Filipi CJ, Walenz T, McBride PJ, Smith SL, et al. Laparoscopic paraesophageal hernia repair. Arch Surg. 1997. 132:586–589.
10. Lund RJ, Wetcher GJ, Raiser F, Glaser K, Perdikis G, Gadenstatter M, et al. Laparoscopic Toupet fundoplication for gastroesophageal reflux disease with poor esophageal body motility. J Gastrointest Surg. 1997. 1:301–308.
11. Horvath KD, Jobe BA, Herron DM, Swanstrom LL. Laparoscopic Toupet fundoplication is an inadequate procedure for patients with severe reflux disease. J Gastrointest Surg. 1999. 3:583–591.
12. Limpert PA, Naunheim KS. Partial versus complete fundoplication: is there a correct answer? Surg Clin North Am. 2005. 85:399–410.
13. Brillantino A, Schettino M, Torelli F, Marano L, Porfidia R, Reda G, et al. Laparoscopic Nissen-Rossetti fundoplication is a safe and effective treatment for both Acid and bile gastroesophageal reflux in patients poorly responsive to proton pump inhibitor. Surg Innov. 2011. 18:387–393.
14. Kaul BK, DeMeester TR, Oka M, Ball CS, Stein HJ, Kim CB, et al. The cause of dysphagia in uncomplicated sliding hiatal hernia and its relief by hiatal herniorrhaphy: a roentgenographic, manometric, and clinical study. Ann Surg. 1990. 211:406–410.
15. Takeyama S, Numata A, Nenohi M, Shibata Y, Okushiba S, Katoh H. Laparoscopic Nissen fundoplication for gastroesophageal reflux disease in Japan. Surg Today. 2004. 34:506–509.
16. Hofstetter WL, Peters JH, DeMeester TR, Hagen JA, DeMeester SR, Crookes PF, et al. Long-term outcome of antireflux surgery in patients with Barrett's esophagus. Ann Surg. 2001. 234:532–538.




 ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download