Abstract
Purpose
Diallyl disulfide (DADS) is a major organosulfur compound derived from garlic. It has been reported that DADS is able to inhibit the proliferation of several tumor cells. In this study, the effect of DADS was investigated in terms of the proliferation of AGS, gastric adenocarcinoma cell line at various concentrations.
Methods
The viability of cultured cells was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide assay. To detect the induction of apoptosis, Annexin V-FITC/propodium iodide (PI) staining assay was performed. Analysis of reactive oxygen species (ROS) and the distribution of cells in the cell cycle were measured by a flow cytometer. And using the Western blot analysis, the change of Fas, caspase-3, Bax, Bcl-2 activity was measured.
Results
The percentage of live AGS cells was decreased to 23% of that in the control group after 400 µM DADS treatment for 48 hours. The Annexin V positive/PI negative (apoptosis portion) area increased from low concentration of DADS to high concentration. When comparing among the DADS treatment groups, the amount of ROS production increased in a dose dependent manner. The percentage of sub-diploid DNA content increased from 8.71% at 50 µM to 25.74% at 400 µM DADS treatment group. The expressions of Fas, caspase-3, Bax were increased and that of Bcl-2 was decreased in a dose dependent manner.
The control of cancer has been focused on chemoprevention that reduces the development of malignancies from precancerous lesions in recent years. Avoiding exposure to cancer causing agents, enhancing host defense mechanisms against cancer, modifying life-styles, and administration of chemopreventive agents are the most common approaches to achieve cancer prevention [1]. Epidemiological studies revealed that high consumption of certain vegetables reduce the risk of variable cancer development including colorectal, stomach, lung, and esophageal cancers [2]. In many studies, dietary advice on preventing cancer has emphasized fruits and vegetables consumption.
Allium vegetables such as garlic, onions, leeks, scallions, chives and shallots are famous for their anti-cancer effect [3]. Among them, garlic is a plant commonly used for seasoning food in many different cultures of the world, especially in Asian countries. Garlic is known to have some medicinal properties, such as anti-hypertensive, anti-hypercholemic and also anti-cancer effects [2,4]. Fresh garlic consists of 60% moisture and 28.60% carbohydrates, 6.40% proteins, 0.01% vitamin C, and 0.07% volatile oils [5]. Some water-soluble and lipid-soluble component can be extracted from condensed steam after the boiling of garlic. The major components of cooked garlic are diallyl disulfide (DADS) and diallyl trisulfide. DADS is a major organosulfur compound derived from garlic and reported to be comprised of approximately 60% garlic oil [6]. Thus, it is the most appropriate compound for use in the evaluation of the possible benefits of garlic. The properties of DADS are divided into anti-oxidation and induction of apoptosis, which is thought to be dependent on the cell type and DADS concentration. Anti-oxidant effects are acquired through increased activity of anti-oxidants such as glutathione peroxidase and induction of apoptosis is acquired from the mechanism associated with intracellular redox environment [7-9]. Moreover, it has been reported that DADS is able to inhibit the proliferation of several tumor cells through characteristics related to apoptosis [10-12]. But, in gastric cancer, the definite anti-cancer effect of DADS and the mechanism of its action has not been established.
Despite the decreased risk factor for the possibility of inducing gastric cancer, the incidence of stomach cancer is highest in Asian people. Considering the consumption of allium vegetables such as garlic is prevalent in Asian countries, the high occurrence of gastric cancer in Asia is contrary to the epigenetic studies on the relationship of garlic ingestion and cancer incidence. To know that garlic has a reversing effect on gastric cancer unlike other cancers or that other molecular effects of garlic affect gastric cancer occurrence, the reliability of DADS for gastric cancer should be investigated. Thus, an attempt to evaluate the effect of DADS, as a major compound of garlic, on gastric cancer cell lines was undertaken. In this study, the effect of DADS was investigated in terms of the proliferation of AGS gastric adenocarcinoma cell lines at various concentrations from low to high levels (0 to 400 µM). The effect of DADS on the induction of apoptosis and production of reactive oxygen species (ROS) was examined to determine the molecular mechanism of DADS. Also, the alteration of cell cycle and change of the intracellular molecular expression were examined.
The human gastric adenocarcinoma cell line, AGS, was purchased from the American Type Culture Collection (CRL-M39, American Type Culture Collection, Manassas, MA, USA). Cells were grown in Dulbecco's minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), at 37℃ in an atmosphere of 5% CO2 in air and routinely trypsinized, plated in 4 × 104/cm2 flasks. Cell viability was assessed by trypan blue exclusion counts.
AGS cells were treated with different amounts of DADS (Sigma, St. Louis, MO, USA) ranging from 0 to 400 µM (0, 50, 100, 200, and 400 µM) at 37℃ in a medium supplemented with serum.
The viability of cultured cells was determined by MTT assay following the reduction of MTT (a tetrazole) to formazan [13]. After 24 hours of incubation with variable concentrations of DADS, AGS cells in 96-well plates were washed twice with phosphate buffered saline (PBS). MTT (100 µg/0.1 mL of PBS) was added to each well. Cells were incubated at 37℃ for 1 hour, and 100 µL of dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. Absorbance was measured at 570 nm with VERSA max tunable microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Cells were incubated with DADS for 48 hours and then harvested. Specific binding of Annexin V-FITC (fluorescein isothiocynate) was performed by incubating the cells for 15 minutes at room temperature in a binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4) containing a saturating concentration of Annexin V-FITC. After incubation, the cells were pelleted and analyzed in a FACScan analyzer (Becton Dickinson, Franklin Lakes, NJ, USA).
DADS treated samples were processed for analysis of ROS by usual flowcytometric techniques. Briefly, cells were harvested, washed twice with PBS and resuspended in a serum-free medium. They were incubated with 50 µmol/L of 2',7'-dichlorofluoroscein-diacetate (DCFH-DA) (Calbiochem, Meudon, France) for 2 hours at 37℃, washed with ice-cold PBS and placed on ice. Fluorescence was measured by FACScan analyzer. As a positive control, cells were separately treated with H2O2 and processed for ROS detection.
Cells were incubated in culture media alone or culture media containing reagents (0, 50, 100, 200, and 400 µM of DADS), at 37℃ for 24 hours. Cells were harvested in cold PBS, fixed in 5 mL of cold 70% ethanol, and stored at 4℃ for subsequent cell cycle analysis. Fixed cells were washed with PBS once and suspended in 1 mL of propodium iodide (PI) staining reagents (20 mg/mL ribonuclease and 5 mg/mL of PI). Samples were incubated at 37℃ in the dark for 30 minutes before cell cycle analysis. The distribution of cells in the cell cycle was measured by a FACScan analyzer.
DADS treated and control cells were rinsed with ice-cold PBS, scraped into 1 mL of PBS and centrifuged at 4,000 rpm for 3 minutes. The pellets were resuspended in radioimmunoprecipitation assay buffer (10 mM Tris. HCl [pH 7.4], 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS] and 1 mM ethylenediaminetetraacetic acid) containing protease inhibitors (0.5 mM phenylmethylsulfonyl fluoride, 10 µg/mL aprotinin and 2 µg/mL of both leupeptin and pepstatin). Then cell extracts were sonicated, and cell debris was removed by centrifugation. Proteins were quantified using the Bradford protein assay kit (BioRad, Hercules, CA, USA) and compared with a γ-globulin standard curve. Equal amounts of total proteins were separated on a SDS-polyacrylamide gel and transferred onto nitrocellulose membranes by electroblotting overnight at 20 V. Membranes were blocked in Tris Buffered Saline-with Tween-20 (TBST) (10 mM Tris-HCl, 150 mM NaCl, and 0.25% Tween 20, pH 7.5) with 5% fat-free powdered milk at room temperature for 1 hour. After the membrane had been rinsed in TBST, the following primary antibodies were used: rabbit or goat polyclonal immunoglobulin (Ig) G for Fas, caspase-3, or mouse monoclonal IgG for Bax, Bcl-2, and β-actin antibodies. After overnight incubation at 4℃ or for 1 hour at room temperature, the membranes were washed four times (10 minutes each) in TBST. The secondary antibodies used were either horseradish peroxidase-conjugated goat anti-rabbit IgG (Cell Signaling, Beverly, MA, USA) or goat anti-mouse IgG (Cell Signaling), followed by five washes with TBST. Bands were detected using ECL substrate (Pierce, Rockford, IL, USA). For β-actin detection, previously probed membranes were soaked in stripping buffer (70 mM Tris. HCl [pH 6.8], 2% SDS, and 0.1% β-mercaptoethanol) at 60℃ for 30 minutes and incubated as above.
All experiments were performed in triplicate and mean ± SD was calculated; representative graphs and figures are shown. Results were analyzed by SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) statistical software. Comparisons between different groups were made by Student's t-test. Statistical significance was defined as P < 0.05.
After adding variable concentrations of DADS, the viability of AGS cells was reduced in a dose-dependent manner. As shown in Fig. 1, the value of OD570 of live AGS cells decreased from 1.42 to 0.33 after DADS treatment for 48 hours with a dose dependent manner. The amount of viable cells decreased to 23% of that in the control group when AGS cells were exposed to 400 µM of DADS for 48 hours. Differences in viable cell count were more evident when at higher concentrations and a statistical significance was shown by comparison of viable cell count between control group and 400 µM DADS treatment group (P < 0.001).
The apoptosis induction was assessed by Annexin V-FITC assay. In the dot plot of flow cytometric analysis (Fig. 2), the lower-right (LR) area was the Annexin V positive/PI negative portion which represented the apoptotic fraction. The percentage of LR (apoptosis portion) area increased gradually according to the concentration of DADS from 2.40% in control group to 9.66% in 400 µM DADS. The difference of induction of apoptosis was prominent between control group and 50 µM DADS treatment group but not significant among the DADS treatment groups (Fig. 3).
In Fig. 4, the mean of fluorescence intensity (MFI) was shown to shift to the right side. Comparing the MFI in variable concentrations of DADS, the change of ROS production was not significantly different between control and low concentration groups (50 µM of DADS). But, at 400 µM of DADS level, ROS production increased significantly. When comparing among the DADS treatment groups, ROS production increased in a dose dependent manner (543.51 at 50 µM to 1,235.69 at 400 µM) considerably (Fig. 5).
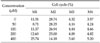
To investigate the alteration of cell cycle caused by DADS, the proportion of cells in each phases was measured with flow cytometric analysis and was gated in histogram with M1 (sub-G1), M2 (G1 phase), M3 (M phase), and M4 (S phase). The M1 gate was indicated the sub-diploid DNA fraction and represented the apoptotic portion. In the histogram of flow cytometric analysis, M1 gate corresponded to the end of the left side of the histogram, and the proportion of M1 increased gradually (Fig. 6). The percentage of each cell cycle is shown in Table 1. Sub-diploid DNA fraction increased from 8.71% at 50 µM to 25.74% at 400 µM (Table 1).
The changes in the proteins associated with apoptosis were evaluated by Western blotting. β-actin expression was monitored to ensure that equal amounts of cytosolic protein samples were loaded. In Fig. 5, the results demonstrated that DADS increased the level of Fas, caspase-3, and Bax in AGS cells, and decreased the level of Bcl-2.
DADS is an oil-soluble organosulfur compound in processed garlic containing a two-sulfur atom. Steam distillation technique yields several organosulfur compounds and the amount of DADS is the greater among them. When ethyl alcohol is used as a solvent, the oxide of DADS called allicin is yielded and at subzero temperature, alliin, an odorless precursor of allicin, is produced [5]. Garlic is known to have several biological properties. Fresh garlic has good bactericidal and anti-thrombotic activity. Inversely, cooked garlic generates principally anti-oxidant effects and anti-tumorigenic effects [13]. In previous reports on garlic ingestion and cancer development, stomach cancer incidence and mortality were lower in the garlic intake group of the amount 1.5 to 7 kg of garlic per year. In china, there was a report on people who ingested 20 g of garlic daily having low incidence of gastric cancer [14]. Among the several organosulfur compounds, lipid solubles such as DADS and diallyl trisulfide are more effective for anti-proliferation of tumor cells than water-soluble compounds. DADS is the most prevalent oil-soluble sulfur compound and is known to inhibit cell proliferation in many cancer cell lines such as, breast, lung, leukemia, neuroblastoma, colon cancer cells [8-11,15]. We used variable concentrations of DADS up to 400 µM. It is possible to extract approximately 0.61 mg of DADS from 1 g of garlic powder, so, the amount of garlic needed for this study is less than 1 g [16]. In in vivo study, the amount needed for a similar effect could differ because the same amount of agent would produce a different biological activity.
Recently, the interest for a dietary treatment by consumption of specific agents with an anti-cancer effect is increasing. Modulating carcinogen activation and induction of apoptosis as elimination of pre-cancerous or cancerous cells selectively are thought to be effective in the control of cancer [17,18]. Agents of induction of apoptosis make good anti-cancer agents because they selectively kill target cells and may have no effect on normal cells [1].
Many studies reported a relationship between the antiproliferative effect of DADS and apoptosis. Apoptosis is a programmed cell death and homeostatic process to remove cells from tissue with minimal disturbance to tissue architecture or function. Apoptosis is related to multicellular organisms and processing phases; evaluating the regulatory molecules and mechanisms is thought to be important to effectively control apoptosis. Studies seeking out agents of apoptosis induction and to evaluate the potential mechanism of apoptosis are increasing.
The mechanism of apoptosis is divided into two pathways. The extrinsic pathway is activated at the cell surface when a specific ligand binds to its corresponding cell-surface death receptor such as Fas. The intrinsic pathway has an apoptotic signal originating from within the cells and relies on the permeabilization of mitochondrial membranes to release apoptogenic mitochondrial proteins [19,20]. Also, in explanation of the mechanism of apoptosis, oxidative stress was thought to play some role. Intracellular redox environment has been suggested to modulate several cellular processes such as gene regulation, cell differentiation, and cell death [21]. In that condition, oxidative stress is implicated in a variety of natural and pathological processes, including apoptosis and several intracellular signaling pathways [22,23]. In this study, we investigated both extrinsic and intrinsic pathways to present the ability of induction apoptosis with DADS and ROS production to evaluate the relationship to oxidative stress.
Fas was used as a death receptor in the extrinsic pathway, and the expression increased in a dose dependent manner. Fas is a member of the death receptor family that signals via formation of a multiprotein complex termed 'death-inducing signaling complex' followed by activation of downstream caspases [24]. In the intracellular molecules related to the mechanism of intrinsic pathway apoptosis, caspase-3, Bax, and Bcl-2 were used. Caspase exists in cells as an inactive precursor form. Apoptotic complex activates the inactive caspase to cleavage product showed at western blot analysis [25]. Bax and Bcl-2 are other intracellular molecules associated with intrinsic pathway of apoptosis. In the Bcl-2 family members, Bax is in the pro-apoptotic family members and Bcl-2 is in the anti-apoptotic family [26]. In this study, the results of Western blot were not clearly different as with the change of DADS concentration. But, compared to the control or low concentration group, in the high concentration group, the activity of Fas, caspase-3, and Bax increased gradually, and Bcl-2 activity decreased (Fig. 7).
The production of ROS increased gradually according to the concentration of DADS, as well. Compared to control group, the ROS production increased slightly in low levels, but came to increase greatly in large amounts of DADS administration. In other previous studies using AGS cell lines, DADS was reported to be ineffective in inducing apoptosis in AGS cell lines because of the characteristics of an unusually high level of gluthathione peroxidase acting as an anti-oxidation enzyme [27]. The concentration of DADS used in that experiment was 50 µM and a definite result of changed induction apoptosis at 100 and 200 µM was not defined. In this study, when experimenting using higher concentrations, significant differences of decreased viability of AGS cells and increased induction of apoptosis and ROS production was reported when compared to lower concentrations. These results showed that the effect of modulation of induction of apoptosis and ROS production was sufficient to overcome the possible anti-oxidative enzyme activity of AGS cell lines in high concentrations of DADS.
Relationships between the cell cycle arrest with peaked G2 phase and apoptosis have been reported in some articles [28]. The cell cycle is composed of four typical phases and regulated well systemically. Between each phase, there is a checkpoint to confirm sufficient preparation of cell progression. Sub-G1 fraction increased gradually as the concentration of DADS increased in this study. These results represent induction of apoptosis. But the sub-G1 increase did not correlate to the degree of the DADS level or ROS production, further study to evaluate the distinct mechanism is needed.
In previous studies, most investigators used low concentrations such as, 10, 25, 50 µM of DADS during short incubation periods, such as 15 minutes to 3 hours [8,10]. Only a few studies have reported the effect of DADS at 100, 200 µM and 24 or 72 hours [12,27]. Yuan et al. [29] reported that 30 µM of DADS induced cell cycle arrest of human gastric cancer MGC803 cells. On the other hand, Filomeni et al. [27] reported the limited anti-proliferative effects of AGS gastric adenocarcinoma cells at 50 µM and the results of 100, 200 µM DADS treatment group were not described clearly in the report. In several other cancer cell lines, high concentrations of about 100, 200, and 500 µM DADS were used. Wu et al. [9] reported the viability of A549 lung carcinoma cells decreased greatly in 200 µM rather than 50, 100 µM of DADS and the amount of induction ROS significantly increased starting from 100 µM and reached a plateau at 200 µM. Variable concentrations, such as, 25, 50, 100, 200, and 400 µM of DADS were used to reveal the effect of DADS to HCT-116 colon cancer cells and the decreased cell viability and increased induction of ROS was noted definitely from 100 µM and showed a maximal level over 200 µM [15].
From these results, the concentration of DADS used in this study was decided from 50 µM to 400 µM. To decide on the proper time to exposure to DADS, we referred to the previous studies. In some studies, ROS production after DADS treatment happened within 30 minutes, reaching peak value at roughly 2 to 3 hours [9,10]. And in the others, the anti-proliferative effect was noted after 24 or 72 hours exposed to DADS. Our incubation time of 48 hours was chosen to obtain the maximal apoptotic effect at individual concentrations. Actually, no reports have been made on the toxic effect of high concentrations of DADS, but, high-density chemicals have an osmotic pressure to some degree and necrosis could affect cell viability. The results showing changes of intracellular molecules such as Fax, caspase-3, Bax, and Bcl-2 in this study were found particularly in high concentration presenting the association of DADS effect with apoptosis rather than necrosis. Further in vivo study to evaluate the possible toxicity of high doses of DADS treatment will be needed.
DADS resulted in an anti-proliferative effect and significant induction of apoptosis in AGS gastric adenocarcinoma cell lines in a dose-dependent manner. These results are related to the increased production of ROS and cell cycle alteration, with an increase of sub-G1 phase percentage. Apoptotic pathway has up-regulatory Fas, caspase-3, Bax activity, and inversely, down-regulatory Bcl-2 activity. In this study, the effect of DADS was revealed prominently at high concentrations, approximately 400 µM. But the relationship of the anti-proliferative effect of DADS and related molecular changes was not clearly proportional to the concentration of DADS. Although the mechanism of anti-cancer effect of DADS could not be clarified, the results that DADS have an antiproliferative effect for AGS gastric cancer cell lines as other cancer cell lines and induces apoptosis, especially in high concentration, and these effects related to ROS production seem to be meaningful.
This study shows that DADS decreases the viability of AGS gastric adenocarcinoma cell lines and induces apoptosis in a dose-dependent manner. These effects might be related to the increases of ROS production. According to the increased DADS concentration, cell cycle alteration and changes of some intracellular molecular activity such as up-regulation of Fas, caspase-3, Bax activity and down-regulation of Bcl-2 was noted. But the relationship of the anti-proliferative effect of DADS and related molecular changes was not clearly proportional to the concentration of DADS, so further study to reveal the distinct mechanism will be needed.
Figures and Tables
Fig. 1
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay of AGS cell viability after diallyl disulfide (DADS) treatment with 48 hours (P-value < 0.001).

Fig. 2
Diallyl disulfide induced apoptosis assessment with FACS analysis of AGS cells stained with Annexin V-FITC and propodium iodide. Apoptotic portion, lower right quadrant.

Fig. 3
The comparison of the results of proportion of apoptotic cells (Annexin V positive/propodium iodide negative cells) with flow cytometric analysis (P-value = 0.045).

Fig. 4
Reactive oxygen species production in AGS cells after treatment of diallyl disulfide with variable concentration.

Fig. 5
Comparison of the reactive oxygen species production between control group and diallyl disulfide (DADS) treatment group of variable concentration. MFI, mean fluorescence intensity.

Fig. 6
The percentage of sub-ciploid DNA-content of cell cycle induced by diallyl disulfide with flow cytometric analysis. M1, sub-diploid fraction and represents apoptotic cells; M2, G0-G1; M3, G2-M; M4, S phase of cells.

References
1. Sun SY, Hail N Jr, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer Inst. 2004. 96:662–672.
2. Fleischauer AT, Arab L. Garlic and cancer: a critical review of the epidemiologic literature. J Nutr. 2001. 131:3S. 1032S–1040S.
3. Wargovich MJ, Uda N, Woods C, Velasco M, McKee K. Allium vegetables: their role in the prevention of cancer. Biochem Soc Trans. 1996. 24:811–814.
4. Milner JA. A historical perspective on garlic and cancer. J Nutr. 2001. 131:3S. 1027S–1031S.
5. Khanum F, Anilakumar KR, Viswanathan KR. Anticarcinogenic properties of garlic: a review. Crit Rev Food Sci Nutr. 2004. 44:479–488.
6. Lawson LD, Wang ZJ. Pre-hepatic fate of the organosulfur compounds derived from garlic (Allium sativum). Planta Med. 1993. 59:A688–A689.
7. Bose C, Guo J, Zimniak L, Srivastava SK, Singh SP, Zimniak P, et al. Critical role of allyl groups and disulfide chain in induction of Pi class glutathione transferase in mouse tissues in vivo by diallyl disulfide, a naturally occurring chemopreventive agent in garlic. Carcinogenesis. 2002. 23:1661–1665.
8. Filomeni G, Aquilano K, Rotilio G, Ciriolo MR. Reactive oxygen species-dependent c-Jun NH2-terminal kinase/ c-Jun signaling cascade mediates neuroblastoma cell death induced by diallyl disulfide. Cancer Res. 2003. 63:5940–5949.
9. Wu XJ, Kassie F, Mersch-Sundermann V. The role of reactive oxygen species (ROS) production on diallyl disulfide (DADS) induced apoptosis and cell cycle arrest in human A549 lung carcinoma cells. Mutat Res. 2005. 579:115–124.
10. Kwon KB, Yoo SJ, Ryu DG, Yang JY, Rho HW, Kim JS, et al. Induction of apoptosis by diallyl disulfide through activation of caspase-3 in human leukemia HL-60 cells. Biochem Pharmacol. 2002. 63:41–47.
11. Nakagawa H, Tsuta K, Kiuchi K, Senzaki H, Tanaka K, Hioki K, et al. Growth inhibitory effects of diallyl disulfide on human breast cancer cell lines. Carcinogenesis. 2001. 22:891–897.
12. Hong YS, Ham YA, Choi JH, Kim J. Effects of allyl sulfur compounds and garlic extract on the expression of Bcl-2, Bax, and p53 in non small cell lung cancer cell lines. Exp Mol Med. 2000. 32:127–134.
13. Abraham KO, Shankaranarayana ML, Raghavan B, Nataraja CP. Alliums-varieties, chemistry, and analysis. Lebenson-Wiss U Technol. 1976. 9:193–200.
14. You WC, Blot WJ, Chang YS, Ershow A, Yang ZT, An Q, et al. Allium vegetables and reduced risk of stomach cancer. J Natl Cancer Inst. 1989. 81:162–164.
15. Song JD, Lee SK, Kim KM, Park SE, Park SJ, Kim KH, et al. Molecular mechanism of diallyl disulfide in cell cycle arrest and apoptosis in HCT-116 colon cancer cells. J Biochem Mol Toxicol. 2009. 23:71–79.
16. Wan X, Polyakova Y, Row KH. Determination of diallyl disulfide in garlic by reversed-phase high performance liquid chromatography. Anal Sci Technol. 2007. 20:442–447.
17. Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997. 278:1073–1077.
18. Omkumar RV, Kadam SM, Banerji A, Ramasarma T. On the involvement of intramolecular protein disulfide in the irreversible inactivation of 3-hydroxy-3-methylglutaryl-CoA reductase by diallyl disulfide. Biochim Biophys Acta. 1993. 1164:108–112.
19. Oez S, Platzer E, Welte K. A quantitative colorimetric method to evaluate the functional state of human polymorphonuclear leukocytes. Blut. 1990. 60:97–102.
20. Hengartner MO. The biochemistry of apoptosis. Nature. 2000. 407:770–776.
21. Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998. 333(Pt 2):291–300.
22. Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997. 88:347–354.
23. Carmody RJ, Cotter TG. Signalling apoptosis: a radical approach. Redox Rep. 2001. 6:77–90.
24. Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003. 10:26–35.
25. Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997. 90:405–413.
26. Ruvolo PP, Deng X, May WS. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia. 2001. 15:515–522.
27. Filomeni G, Aquilano K, Rotilio G, Ciriolo MR. Glutathione-related systems and modulation of extracellular signal-regulated kinases are involved in the resistance of AGS adenocarcinoma gastric cells to diallyl disulfide-induced apoptosis. Cancer Res. 2005. 65:11735–11742.
28. Herman-Antosiewicz A, Powolny AA, Singh SV. Molecular targets of cancer chemoprevention by garlic-derived organosulfides. Acta Pharmacol Sin. 2007. 28:1355–1364.
29. Yuan JP, Wang GH, Ling H, Su Q, Yang YH, Song Y, et al. Diallyl disulfide-induced G2/M arrest of human gastric cancer MGC803 cells involves activation of p38 MAP kinase pathways. World J Gastroenterol. 2004. 10:2731–2734.




 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download