Abstract
Purpose
Interleukin (IL)-9 induces allergic responses; however, the roles of anti-IL-9 antibody in the induction of tolerance remain unclear. This study investigated the effects of anti-IL-9 antibody on oral tolerance (OT) in a mouse model of allergic rhinitis (AR).
Methods
BALB/c mice were divided into 4 groups: the control, AR, OT, and OT with anti-IL-9 antibody (OT+IL9AB) groups. Ovalbumin (OVA) was used for sensitization and challenge. Mice in the OT and OT+IL9AB groups were fed OVA for immunotherapy. During immunotherapy, OT+IL9AB mice were injected with anti-IL-9 antibody. Allergic symptoms, tissue eosinophil counts, and serum OVA-specific immunoglobulin E (IgE) were measured. The mRNA expressions of cytokines and transcription factors of T cells of nasal mucosa were determined by real-time polymerase chain reaction (PCR). The protein levels of GATA3, ROR-γt, and Foxp3 in nasal mucosa were determined by Western blot. CD4+CD25+Foxp3+ T cells in the spleen were analyzed by flow cytometry.
Results
Administration of anti-IL-9 antibody decreased allergic symptoms, OVA-specific IgE levels, and eosinophil counts. In addition, it inhibited T-helper (Th) 2 responses, but had no effect on Th1 responses. Protein levels of ROR-γt and mRNA levels of PU.1 and ROR-γt were reduced by anti-IL-9 antibody. Anti-IL-9 antibody increased Foxp3 and IL-10 mRNA expression, Foxp3 protein, and induction of CD4+CD25+Foxp3+ T cells.
Conclusions
Anti-IL-9 antibody decreased allergic inflammation through suppression of Th2 and Th17 cells. Anti-IL-9 antibody enhanced the tolerogenic effects of regulatory T cells. These results suggest that anti-IL-9 antibody might represent a potential therapeutic agent for allergen immunotherapy in patients with uncontrolled allergic airway disease.
Allergic rhinitis (AR) affects 1 billion people worldwide, with a 15%-20% prevalence that is increasing rapidly. Many patients with AR have insufficient relief of symptoms and seek anti-allergic medications as the first treatment.123 Allergen immunotherapy is an effective and fundamental treatment for allergic diseases, such as asthma and AR. However, more advanced strategies are needed in terms of effectiveness and safety.45 In this regard, recent treatment improvements include a combination of allergen immunotherapy and biological modifiers, such as therapeutic antibodies, receptors, cytokines, and small molecules.6
Allergic diseases, such as AR and asthma, are propelled mainly by T-helper (Th) 2 cells.7 However, recent studies have implicated several other helper T cells involved in allergic inflammation including Th17, Th9, Th22, and Th25 cells.8 Th9 cells are generated in the presence of interleukin (IL)-4 and transforming growth factor (TGF)-β, and are major contributors to allergic disease through IL-9.9 Besides Th9 cells, IL-9 is produced by multiple sources, such as type 2 innate lymphoid cells (ILC2s), mast cells, eosinophils, natural killer (NK)-T cells, Th2 cells, Th17 cells, and regulatory T (Treg) cells.1011 IL-9 has pathophysiological functions in allergy, autoimmune disease, and leukemia as well as physiological functions in nematode infection and melanoma.12
IL-9 was initially considered a Th2 cytokine; however, now it is known to be produced by Th9 cells. IL-9, together with IL-4, IL-5, and IL-13, is a cytokine of the type 2 immune response that induces allergic inflammation.13 IL-9 plays multiple roles, acting as a growth factor for T cells, enhancing immunoglobulin E (IgE) production, inducing mucus production by epithelial cells, and promoting tissue mast cell accumulation.1415 In a mouse model of asthma, administration of anti-IL-9 antibody reduced allergic pathologies.16 In human studies, anti-IL-9 monoclonal antibody in adults with mild to moderate asthma showed acceptable safety profiles and promising clinical activities, although it showed no improvement in symptoms in adults with uncontrolled asthma.1718 Based on these reports, we hypothesized that anti-IL-9 antibody could be considered an adjuvant in allergen immunotherapy. However, few studies have evaluated the effects of IL-9 on tolerance induction in allergic inflammation.
Oral tolerance (OT) has been defined as the specific suppression of immune response to a specific antigen by oral administration of the antigen.19 OT has been studied in treatment of autoimmune and allergic diseases.2021 Many studies using OT in allergic disease have shown the suppression of Th2 responses and induction of Treg cells.22
Therefore, we investigated the effect of anti-IL-9 antibody on OT in a mouse model of AR. After sensitization, OT was induced by feeding with ovalbumin (OVA). During OT, 1 group of mice was injected with anti-IL-9 antibody. The effects of OT with or without administration of anti-IL-9 antibody were compared. To the best of our knowledge, this is the first study to evaluate the influence of anti-IL-9 antibody on OT in a mouse model of AR.
Six-week-old female BALB/c mice weighing 20-30 g were used in this study. The experiment was performed with the approval of the Institutional Animal Care and Use Committee at The Catholic University of Korea.
Forty mice were randomized to 1 of 4 groups: the control (n=10), AR (n=10), OT (n=10), and OT with anti-IL-9 antibody (OT+IL9AB) (n=10) groups. Allergen sensitization and challenge protocols are summarized in Fig. 1. Briefly, on days 0, 7, and 14, all mice except those in the control group were immunized with an intraperitoneal injection of OVA 25 µg (grade V; Sigma-Aldrich, St. Louis, MO, USA) and aluminum hydroxide 1 mg (Aldrich, Milwaukee, WI, USA). On days 28-36, mice in the OT and OT+IL9AB groups were fed 20 mg of OVA 5 times to induce tolerance. During this period, mice in the OT+IL9AB group were intraperitoneally injected with 500 µg of purified anti-mouse IL-9 antibody (clone MM9C1; BioLegend, San Diego, CA, USA),23 while those in the OT group were injected with isotype IgG. All sensitized mice were challenged intranasally with 50 µg of OVA on days 43-49. The control group received phosphate-buffered saline (PBS) intranasally instead of OVA. Fig. 1 depicts the protocol used in this study.
The number of sneezing and nose-rubbing motions during 15 minutes after the final allergen challenge was recorded and compared among the experimental groups. The assessors were blinded to the protocol.
Blood samples were collected from mice 24 hours after the last challenge, and the sera were separated by centrifugation. The serum OVA-specific IgE level was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Pharmingen, San Diego, CA, USA).
The decapitated heads were fixed in paraformaldehyde, decalcified with Calci-Clear Rapid (National Diagnostics, Atlanta, GA, USA) and embedded in paraffin. The blocks were sliced into 4-µm-thick sections and stained with hematoxylin and eosin (H & E) to evaluate general morphology and number of eosinophils in the lamina propria. The average number of eosinophils was counted in 4 areas around the nasal septa under a light microscope. The individual who counted the eosinophils was blinded to the protocol.
Nasal mucosa was removed to evaluate the level of mRNA expression using real-time PCR. Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) from nasal mucosa, and the first strand was reverse-transcribed using a random primer (TaKaRa, Otsu, Japan). The oligonucleotide primer sequences were as follows: interferon (IFN)-γ forward primer, 5′-AGAGCCAGATTATCTCTTTCTACCTCAG-3′ and IFN-γ reverse primer, 5′-CCTTTTTCGCCTTGCTGTTG-3′; T-bet forward primer, 5′-GCCAGGGAACCGCTTATA-3′ and T-bet reverse primer, 5′-CCTTGTTGTTGGTGAGCTTTA-3′; IL-4 forward primer, 5′-TCAACCCCCAGCTAGTTGTC-3′ and IL-4 reverse primer, 5′-AAATATGCGAAGCACCTTGG-3′; GATA3 forward primer, 5′-CTGGATGGCGGCAAAGC-3′ and GATA3 reverse primer, 5′-GTGGGCGGGAAGGTGAA-3′; IL-17 forward primer, 5′-TCCAGAAGGCCCTCAGACTA-3′ and IL-17 reverse primer, 5′-AGCATCTTCTCGACCCTGAA-3′; ROR-γt forward primer, 5′-AGCATCTATAGCACTGACGG-3′ and ROR-γt reverse primer, 5′-CAGAAACTGGGAATGCAGTG-3′; PU.1 forward primer, 5′-GCATCTGGTGGGTGGACAA-3′ and PU.1 reverse primer, 5′-TCTTGCCGTAGTTGAG-3′; IL-10 forward primer, 5′-GCTCTTACTGACTGGCATGAG-3′ and IL-10 reverse primer, 5′-CGCAGCTCTAGGAGCATGTG-3′; TGF-β forward primer, 5′-CACCATCCATGACATGAACC-3′, and TGF-β reverse primer 5′-TCATGTTGGACAACTGCTCC-3′; Foxp3 forward primer, 5′-GAAAGCGGATACCAAATGA-3′ and Foxp3 reverse primer, 5′-CTGTGAGGACTACCGAGCC-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward primer, 5′-GCACAGTCAAGGCCGAGAAT-3′ and GAPDH reverse primer, 5′-GCCTTCTCCATGGTGGTGAA-3′. The expression levels of IFN-γ, IL-4, T-bet, GATA3, PU.1, IL-17, ROR-γt, IL-10, TGF-β, Foxp3, and GAPDH mRNA were determined by real-time PCR using the ABI PRISM 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) and SYBR Green PCR master mix (TaKaRa). The expression levels of these mRNAs were analyzed using the ABI 7300 Sequence Detection System (Applied Biosystems). The results were normalized to β-actin expression and represented as the fold-increase with respect to expression in the control group.
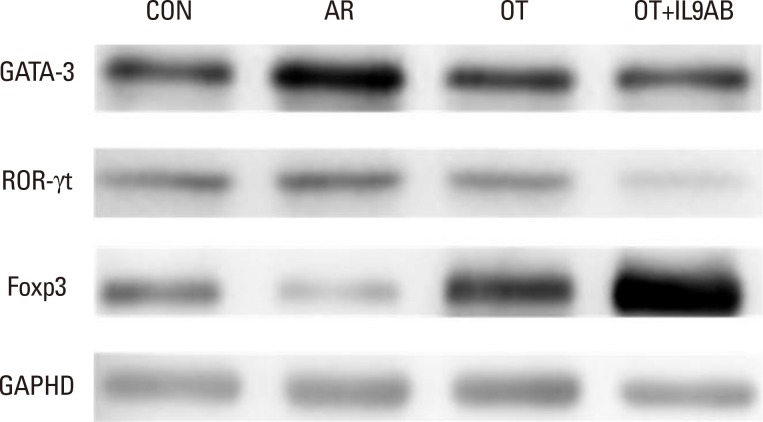
The protein levels of GATA3, ROR-γt, and Foxp3 in nasal mucosa were determined by Western blot. The membrane was probed with antibodies against GATA3, ROR-γt, Foxp3, and GAPDH as the normal control. Anti-GATA3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-ROR-γt (eBioscience, San Diego, CA, USA), and anti-Foxp3 (eBioscience) antibodies were used.
The spleen was removed aseptically 24 hours after the final challenge. For cell surface staining, 106 splenic mononuclear cells were incubated with fluorescein isothiocyanate (FITC)-conjugated mouse CD4 (GK1.5) antibody (eBioscience). For intracellular staining, cells stained with CD4 were incubated with fixation/permeabilization working solution, and Fc receptors were blocked with excess mouse Fc block. Cells were then stained with phycoerythrin (PE)-Cy5-conjugated mouse Foxp3 (FJK-16s) and APC-CD25 antibodies (eBioscience). CD4+CD25+Foxp3+ T cells were analyzed using FACS-Calibur (Becton Dickinson, San Jose, CA, USA).
All measured parameters are expressed as means±standard deviation (SD). The differences among the groups were analyzed using Kruskal-Wallis analysis. In cases with statistical differences, the ranked parameters were compared using one-way analysis of variance (ANOVA) and Bonferroni's multiple comparison tests. Analyses were performed with PASW Statistics software (ver. 18.0; SPSS Inc., Chicago, IL, USA). A P value of < 0.05 was considered to indicate statistical significance.
To determine the effect of anti-IL-9 antibody on allergy symptoms, we evaluated the number of sneezing and rubbing motions during 15 minutes after the final challenge. Mice in the OT and OT+IL9AB groups had lower numbers of sneezing motions than those in the AR group (OT vs AR, P=0.001; OT+IL9AB vs AR, P=0.003). The number of sneezing motions in the OT+IL9AB group was significantly decreased compared with that in the OT group (P=0.04; Fig. 2A). The number of nasal rubbings was higher in the OT and OT+IL9AB groups than in the AR groups (OT vs AR, P=0.000; OT+IL9AB vs AR, P=0.001). The number of nasal rubbings in the OT+IL9AB group was also diminished compared with that in the OT group, although no significant difference was detected (Fig. 2B). We found that administration of anti-IL-9 antibody during induction of OT decreased allergy symptoms.
Serum OVA-specific IgE levels indicate the status of atopy. Serum OVA-specific IgE levels were elevated markedly in the AR group compared with the OT and OT+IL9AB groups (AR vs OT, P=0.002; AR vs OT+IL9AB, P=0.006). The OT+IL9AB group displayed significantly lower serum OVA-specific IgE than the OT group, indicating that anti-IL-9 antibody decreases OVA-specific IgE synthesis (P=0.010, Fig. 3A).
As eosinophils are a key player in allergic inflammation, we evaluated whether anti-IL-9 antibody could reduce eosinophil infiltration. The AR group had a higher eosinophil count than the OT and OT+IL9AB groups (AR vs OT, P=0.002; AR vs OT+IL9AB; P=0.005). In addition, we found that the eosinophil count was lower in the OT+IL9AB group than in the OT group (P=0.049). These results show that anti-IL-9 antibody during OT induction attenuates eosinophil infiltration in nasal mucosa (Fig. 3B). Fig. 3C shows the infiltration of eosinophils in the lamina propria.
As the imbalance of Th1 and Th2 responses causes allergy inflammation, we evaluated whether anti-IL-9 antibody could restore this imbalance. The mRNA expressions of IFN-γ (a Th1 cytokine) and T-bet (a transcription factor for Th1 cells) were increased in the AR group compared with the other groups, although without significance. No difference in IFN-γ and T-bet mRNA expression was detected between the OT and the OT+IL9AB groups (Fig. 4A and B). In contrast, the mRNA expression of IL-4 (a Th2 cytokine) was increased significantly in the AR group compared with the OT and OT+IL9AB groups (AR vs OT, P=0.013; AR vs OT+IL9AB, P=0.042). Likewise, the mRNA expression of GATA3 (a transcription factor for Th2 cells) was significantly increased in the AR group (AR vs OT, P=0.005; AR vs OT+IL9AB, P=0.001). The OT+IL9AB group displayed decreased mRNA expressions of IL-4 (P=0.049) compared with the OT group (Fig. 4C). The mRNA expression of GATA3 was also decreased in the OT+IL9AB group compared with the OT group, although no significant difference detected (Fig. 4D). Western blot analysis showed lower GATA3 protein levels in the OT and OT+IL9AB groups compared with those in the AR group, with the OT+IL9AB group showing lower GATA3 protein levels than the OT group (Fig. 5). These results demonstrated that administration of anti-IL-9 antibody during OT induction suppressed Th2 responses, but had little effect on Th1 responses.
Th9 and Th17 cells are recently identified contributors to allergic inflammation. Th9 cells are involved in mucus hyperplasia, mast cell accumulation, lung remodeling, and airway hyper-reactivity via production of IL-9.10 Th17 cells, a newly defined subpopulation of CD4+ T cells, develop airway neutrophilia via production of IL-17 in allergic airways.8 We evaluated the effect of anti-IL-9 antibody on both the mRNA expression of PU.1 (a transcription factor for Th9 cells) and ROR-γt (a transcription factor for Th17 cells). PU.1 mRNA expression was higher in the AR group than in the OT or OT+IL9AB groups (AR vs OT, P=0.001; AR vs OT+IL9AB, P=0.001). The OT+IL9AB group had significantly lower PU.1 mRNA expression than the OT group (P=0.004, Fig. 6A). Mice in the AR group had significantly higher expression of ROR-γt and IL-17 mRNA than those in the OT and OT+IL9AB groups. ROR-γt mRNA levels was decreased in the OT+IL9AB group compared with the OT group, similar to PU.1 mRNA expression (P=0.047), whereas no significant difference of IL-17 mRNA levels was detected between the OT and OT+IL9AB groups (Fig. 6B and C). Western blot analysis showed that the ROR-γt protein level was decreased primarily in the OT+IL9AB group (Fig. 5). These results showed that anti-IL-9 antibody negatively affects the induction of Th9 and Th17 cells.
Treg cells have tolerogenic effects in allergic inflammation via production of IL-10 and TGF-β, inhibitory cytokines of the Th2 response.24 Moreover, the presence of TGF-β affects production of IL-9.25 We evaluated whether anti-IL-9 antibody could potentiate OT in our mouse model. The mRNA expressions of Foxp3 (a transcription factor for Treg cells) and IL-10 were reduced in the AR group compared the OT and OT+IL9AB groups (Foxp3: AR vs OT, P=0.026; AR vs OT+IL9AB, P=0.001. IL-10: AR vs OT, P=0.001; AR vs OT+IL9AB, P=0.000). The OT+IL9AB group had significantly higher mRNA expression of Foxp3 (P=0.015) and IL-10 (P=0.049) than the OT group (Fig. 7A and B). TGF-β was lower in the AR group than in the OT group (P=0.001). In contrast to the IL-10 mRNA level, the TGF-β mRNA level was decreased in the OT+IL9AB group compared with the OT group (P=0.001, Fig. 7B and C). Western blot analysis showed increased Foxp3 protein levels in the OT and OT+IL9AB groups compared with the AR group. The OT+IL9AB group showed markedly higher levels of Foxp3 protein than the OT group (Fig. 5). These results supported that anti-IL-9 antibody increased tolerance induction in this mouse model despite decreased TGF-β mRNA levels.
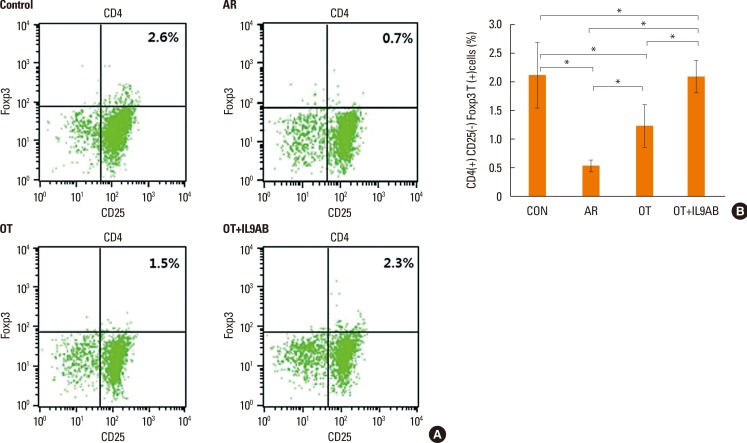
Cells were sorted based on their level of Foxp3 and CD25 expression and whether they expressed CD4 (Fig. 8A). The percentage of CD4+CD25+Foxp3+ T cells is a proportion of total splenic mononuclear cells. The AR group had lower percentages of these cells than the OT and OT+IL9AB groups (AR vs OT, P=0.022; AR vs OT+IL9AB, P=0.015). Similar to the result of Foxp3 mRNA expression, the percentage of these cells was increased significantly in the OT+IL9AB group compared with the OT group (P=0.031, Fig. 8B). These results indicate that administration of anti-IL-9 antibody produced an increase in CD4+CD25+Foxp3+ T cells during tolerance induction.
IL-9 is a pleiotropic cytokine that plays a role in allergic inflammation. In asthma, IL-9 increases ILC2 survival, Th2 cell responses, IgE production, and innate cell inflammation. In addition, IL-9 induces collagen deposition, smooth muscle hyperplasia, and mucus production.10 However, the precise mechanisms of allergic inflammation by IL-9 and the effects of IL-9 on tolerance induction are unclear.
OT has been used in many studies using animal models of allergic diseases. In mouse models of AR or asthma, feeding allergen suppressed airway reactivity, airway eosinophilia, and production of Th2 cytokines and allergen-specific IgE. It also induced the production of regulatory cytokines secreted by Treg cells.26272829 Therefore, OT, a type of mucosal tolerance, may be considered a strategy of allergen-specific immunotherapy.
This study examined the effects of anti-IL-9 antibody on OT in a mouse model of AR. We demonstrated that anti-IL-9 antibody suppressed allergic inflammation by inhibiting the induction of Th2 and Th17 cells. Furthermore, we found that anti-IL-9 antibody enhanced induction of Treg cells and production of IL-10, a tolerogenic cytokine, but decreased the production of TGF-β. These results suggest that neutralizing IL-9 may increase the induction of OT in a mouse model of AR.
Many murine studies have demonstrated that IL-9 mediates allergic inflammation. IL-9 is associated with the development of airway hyper-responsiveness (AHR) and airway remodeling.3031 In the present study, administration of anti-IL-9 antibody reduced the sneezing response. In addition, serum OVA-specific IgE and eosinophil infiltration in nasal mucosa were decreased by neutralization of IL-9. GATA3 and IL-4 mRNA expression, as well as GATA3 protein levels, were also reduced by the administration of anti-IL-9 antibody. Our results were similar to those from previous studies using mouse models of atopic disease, which showed that Th9 cells promote mast and eosinophil accumulation, mucus production, Th2 cytokine production, and bronchial hyperresponsiveness via production of IL-9. These effects are reversed by the transfer of Il9-/-Th9 cells or by IL-9 neutralization.1623323334 In a model of chronic airway hyperreactivity, the number of Th9 cells directly correlated with the severity of AHR, and anti-IL-9 treatment decreased airway inflammation.23 Another study using a mouse model reported that IL-9 neutralization reversed airway remodeling via reduction of mast cell numbers and decreased expression of the profibrotic mediators.32 In a study of mice with PU.1-deficient T cells, the expression of IL-9 and chemokines was reduced and allergic inflammation was attenuated.33 In addition, IL-9 neutralization reduced infiltration of inflammatory cells and their cytokine production in murine asthma models.34
Many studies have reported that IL-9 mediates allergic inflammation in human, although the precise mechanism of pathogenesis by IL-9 remains unclear. In previous study, allergic patients had elevated Th9 cells in peripheral blood mononuclear cells together with elevated serum IgE levels.35 A recent study showed that serum IL-9 concentrations in patients with allergic asthma were increased and inversely related to eosinophil apoptosis.36 Another study reported that IL-9 gene expression in serum samples was increased in asthmatics, which was correlated with disease severity.37 A study of atopic infants demonstrated that IL-9 production from allergen-stimulated peripheral blood mononuclear cells was increased in the absence of concomitant increases in the production of other Th2 cytokines, suggesting that IL-9 may be produced earlier than other cytokines in the development of atopic disease.38 Two different studies with allergic patients demonstrated that Th9 differentiation and IL-9 production in allergic inflammation was promoted by thymic stromal lymphopoietin, an epithelial cell-derived cytokine.3940
As PU.1 is a regulator of IL-9 production in T cells,4142 anti-IL-9 antibody could reduce PU.1 mRNA expression. In an in vitro study, IL-9 with TGF-β enhanced differentiation of CD4+ T cells into Th17 cells.43 In a murine asthma model, anti-IL-9 antibody treatment inhibited airway inflammation by reducing the number of Th17 cells and IL-17 levels.34 In an experimental autoimmune encephalomyelitis (EAE) murine model, IL-9 blockade with anti-IL-9 antibody reduced both the production of IL-17 and development of EAE.44 Our results were consistent with those of previous studies. We demonstrated that anti-IL-9 antibody inhibited the mRNA expression of both PU.1 and ROR-γt and reduced the protein level of ROR-γt. These results showed a negative effect of anti-IL-9 antibody on the induction of both Th9 and Th17 cells, which are major contributors to allergic inflammation.
The main aim of this study was to examine the effect of anti-IL-9 antibody on tolerance induction in allergic inflammation. There have been conflicting data from studies investigating the effects of IL-9 on Treg in different diseases. In a mouse model of allograft tolerance, IL-9 induced mast cell recruitment and activation essential for the immunosuppressive effect of Treg cells.45 In the EAE model, mice lacking the IL-9 receptor had a defect in the suppressive activity of Treg cells,43 whereas IL-9-deficient mice had increased numbers of Treg cells in the spinal cord.46 In a rat model of experimental autoimmune myasthenia gravis (EAMG), neutralization of IL-9 inhibited the pathology of EAMG by reducing the number of Th1 cells and increasing the number of Treg cells.47 In a murine model of nephrotoxic serum nephritis (NTS), blockage of IL-9 reduced protection from NTS by both Treg and mast cells. However, IL-9 deficiency had little effect on the general suppressive activity of Treg cells.48 These discrepancies may be caused by differences in elicited diseases and roles of IL-9 on different cells with IL-9 receptors in various immune environments. However, few studies have examined the effect of IL-9 on Treg cells in allergic inflammation. The present study showed that anti-IL-9 antibody increased induction of tolerance by Treg cells. Induction of Treg cells and production of cytokines produced by Treg cells were increased by the administration of anti-IL-9 antibody. On the other hand, anti-IL-9 antibody reduced TGF-β mRNA levels, as in a previous study, using a murine model with chronic airway inflammation, which showed that anti-IL-9 antibody-treated mice had decreased expression of TGF-β in the lung and reduced pathology of airway remodeling.32 Therefore, administration of anti-IL-9 antibody may prevent airway remodeling including fibrosis. These results supported the notion that anti-IL-9 antibody can be a novel treatment target for allergic airway diseases such as asthma and AR.
However, the current study had several limitations. First, the number of animals included in this study was small. Second, this study lacked measurement of IL-9 levels in nasal mucosa. Nevertheless, this study is informative because, as far as we know, this is the first study to evaluate the influence of anti-IL-9 antibody on OT in a mouse model of AR.
In summary, this study demonstrated the tolerogenic potentials of anti-IL-9 antibody on OT in a mouse model of AR. Anti-IL-9 antibody decreased allergic inflammation through suppression of Th2 and Th17 cells. Our study showed that anti-IL-9 antibody enhanced Treg cell induction and the production of tolerogenic cytokines by Treg cells. These results suggest that anti-IL-9 antibody might be a potential immunotherapeutic target in allergen immunotherapy for patients with uncontrolled allergic airway disease.
ACKNOWLEDGMENTS
The work was supported by the Alumni Association of the Department of Otolaryngology-Head and Neck Surgery, College of Medicine, The Catholic University of Korea, and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2011-0026915).
References
1. Price D, Smith P, Hellings P, Papadopoulos N, Fokkens W, Muraro A, et al. Current controversies and challenges in allergic rhinitis management. Expert Rev Clin Immunol. 2015; 11:1205–1217. PMID: 26325631.

2. Ciprandi G, Marseglia GL, Castagnoli R, Valsecchi C, Tagliacarne C, Caimmi S, et al. From IgE to clinical trials of allergic rhinitis. Expert Rev Clin Immunol. 2015; 11:1321–1333. PMID: 26358006.

3. Kim CH, Kim JK, Kim HJ, Cho JH, Kim JS, Kim YD, et al. Comparison of intranasal ciclesonide, oral levocetirizine, and combination treatment for allergic rhinitis. Allergy Asthma Immunol Res. 2015; 7:158–166. PMID: 25729623.

4. Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013; 131:1288–1296.e3. PMID: 23498595.
5. Jutel M, Kosowska A, Smolinska S. Allergen immunotherapy: past, present, and future. Allergy Asthma Immunol Res. 2016; 8:191–197. PMID: 26922928.

6. Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012; 18:736–749. PMID: 22561837.

7. Kim YM, Kim YS, Jeon SG, Kim YK. Immunopathogenesis of allergic asthma: more than the Th2 hypothesis. Allergy Asthma Immunol Res. 2013; 5:189–196. PMID: 23814671.

8. Pawankar R, Hayashi M, Yamanishi S, Igarashi T. The paradigm of cytokine networks in allergic airway inflammation. Curr Opin Allergy Clin Immunol. 2015; 15:41–48. PMID: 25479317.

9. Wisniewski JA, Borish L. Novel cytokines and cytokine-producing T cells in allergic disorders. Allergy Asthma Proc. 2011; 32:83–94. PMID: 21439160.

10. Kaplan MH, Hufford MM, Olson MR. The development and in vivo function of T helper 9 cells. Nat Rev Immunol. 2015; 15:295–307. PMID: 25848755.

11. Wilhelm C, Turner JE, Van Snick J, Stockinger B. The many lives of IL-9: a question of survival? Nat Immunol. 2012; 13:637–641. PMID: 22713829.

12. Schmitt E, Klein M, Bopp T. Th9 cells, new players in adaptive immunity. Trends Immunol. 2014; 35:61–68. PMID: 24215739.

13. Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015; 15:271–282. PMID: 25882242.

14. Sehra S, Yao W, Nguyen ET, Glosson-Byers NL, Akhtar N, Zhou B, et al. TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J Allergy Clin Immunol. 2015; 136:433–440.e1. PMID: 25746972.

15. Boyman O, Kaegi C, Akdis M, Bavbek S, Bossios A, Chatzipetrou A, et al. EAACI IG biologicals task force paper on the use of biologic agents in allergic disorders. Allergy. 2015; 70:727–754. PMID: 25819018.

16. Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010; 33:192–202. PMID: 20674401.

17. Oh CK, Leigh R, McLaurin KK, Kim K, Hultquist M, Molfino NA. A randomized, controlled trial to evaluate the effect of an anti-interleukin-9 monoclonal antibody in adults with uncontrolled asthma. Respir Res. 2013; 14:93. PMID: 24050312.

18. Parker JM, Oh CK, LaForce C, Miller SD, Pearlman DS, Le C, et al. Safety profile and clinical activity of multiple subcutaneous doses of MEDI-528, a humanized anti-interleukin-9 monoclonal antibody, in two randomized phase 2a studies in subjects with asthma. BMC Pulm Med. 2011; 11:14. PMID: 21356110.

20. Sato MN, Carvalho AF, Silva AO, MacIel M Jr, Fusaro AE, Duarte AJ. Oral tolerance induced to house dust mite extract in naive and sensitized mice: evaluation of immunoglobulin G anti-immunoglobulin E autoantibodies and IgG-IgE complexes. Immunology. 1998; 95:193–199. PMID: 9824475.

21. Strobel S. Oral tolerance, systemic immunoregulation, and autoimmunity. Ann N Y Acad Sci. 2002; 958:47–58. PMID: 12021083.

22. Ruiz Schütz VC, Drewiacki T, Nakashima AS, Arantes-Costa FM, Prado CM, Kasahara DI, et al. Oral tolerance attenuates airway inflammation and remodeling in a model of chronic pulmonary allergic inflammation. Respir Physiol Neurobiol. 2009; 165:13–21. PMID: 18930843.

23. Kerzerho J, Maazi H, Speak AO, Szely N, Lombardi V, Khoo B, et al. Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity. J Allergy Clin Immunol. 2013; 131:1048–1057. 1057.e1–1057.e2. PMID: 23174661.

24. Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J. 2015; 8:17. PMID: 26023323.

25. Nowak EC, Noelle RJ. Interleukin-9 and T cell subsets. Cell Cycle. 2009; 8:3798–3799. PMID: 19934658.

26. Chung Y, Cho J, Chang YS, Cho SH, Kang CY. Preventive and therapeutic effects of oral tolerance in a murine model of asthma. Immunobiology. 2002; 206:408–423. PMID: 12437071.

27. Keller AC, Mucida D, Gomes E, Faquim-Mauro E, Faria AM, Rodriguez D, et al. Hierarchical suppression of asthma-like responses by mucosal tolerance. J Allergy Clin Immunol. 2006; 117:283–290. PMID: 16461128.

28. Shin JH, Kang JM, Kim SW, Cho JH, Park YJ, Kim SW. Effect of oral tolerance in a mouse model of allergic rhinitis. Otolaryngol Head Neck Surg. 2010; 142:370–375. PMID: 20172383.

29. Adel-Patient K, Wavrin S, Bernard H, Meziti N, Ah-Leung S, Wal JM. Oral tolerance and Treg cells are induced in BALB/c mice after gavage with bovine β-lactoglobulin. Allergy. 2011; 66:1312–1321. PMID: 21615416.

30. Farahani R, Sherkat R, Hakemi MG, Eskandari N, Yazdani R. Cytokines (interleukin-9, IL-17, IL-22, IL-25 and IL-33) and asthma. Adv Biomed Res. 2014; 3:127. PMID: 24949298.

31. van den Brûle S, Heymans J, Havaux X, Renauld JC, Lison D, Huaux F, et al. Profibrotic effect of IL-9 overexpression in a model of airway remodeling. Am J Respir Cell Mol Biol. 2007; 37:202–209. PMID: 17446528.

32. Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, et al. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am J Respir Crit Care Med. 2011; 183:865–875. PMID: 20971830.

33. Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010; 11:527–534. PMID: 20431622.

34. Kim MS, Cho KA, Cho YJ, Woo SY. Effects of interleukin-9 blockade on chronic airway inflammation in murine asthma models. Allergy Asthma Immunol Res. 2013; 5:197–206. PMID: 23814672.

35. Jones CP, Gregory LG, Causton B, Campbell GA, Lloyd CM. Activin A and TGF-beta promote T(H)9 cell-mediated pulmonary allergic pathology. J Allergy Clin Immunol. 2012; 129:1000–1010.e3. PMID: 22277204.
36. Hoppenot D, Malakauskas K, Lavinskienė S, Bajoriūnienė I, Kalinauskaitė V, Sakalauskas R. Peripheral blood Th9 cells and eosinophil apoptosis in asthma patients. Medicina (Kaunas). 2015; 51:10–17. PMID: 25744770.

37. Sherkat R, Yazdani R, Ganjalikhani Hakemi M, Homayouni V, Farahani R, Hosseini M, et al. Innate lymphoid cells and cytokines of the novel subtypes of helper T cells in asthma. Asia Pac Allergy. 2014; 4:212–221. PMID: 25379481.

38. Yao W, Tepper RS, Kaplan MH. Predisposition to the development of IL-9-secreting T cells in atopic infants. J Allergy Clin Immunol. 2011; 128:1357–1360.e5. PMID: 21798577.

39. Froidure A, Shen C, Gras D, Van Snick J, Chanez P, Pilette C. Myeloid dendritic cells are primed in allergic asthma for thymic stromal lymphopoietin-mediated induction of Th2 and Th9 responses. Allergy. 2014; 69:1068–1076. PMID: 24888572.

40. Yao W, Zhang Y, Jabeen R, Nguyen ET, Wilkes DS, Tepper RS, et al. Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity. 2013; 38:360–372. PMID: 23376058.

41. Licona-Limón P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013; 14:536–542. PMID: 23685824.

42. Hoppenot D, Malakauskas K, Lavinskiene S, Sakalauskas R. p-STAT6, PU.1, and NF-kappaB are involved in allergen-induced late-phase airway inflammation in asthma patients. BMC Pulm Med. 2015; 15:122. PMID: 26466682.

43. Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009; 106:12885–12890. PMID: 19433802.

44. Li H, Nourbakhsh B, Ciric B, Zhang GX, Rostami A. Neutralization of IL-9 ameliorates experimental autoimmune encephalomyelitis by decreasing the effector T cell population. J Immunol. 2010; 185:4095–4100. PMID: 20805418.

45. Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006; 442:997–1002. PMID: 16921386.

46. Li H, Nourbakhsh B, Cullimore M, Zhang GX, Rostami A. IL-9 is important for T-cell activation and differentiation in autoimmune inflammation of the central nervous system. Eur J Immunol. 2011; 41:2197–2206. PMID: 21674475.

47. Yao X, Kong Q, Xie X, Wang J, Li N, Liu Y, et al. Neutralization of interleukin-9 ameliorates symptoms of experimental autoimmune myasthenia gravis in rats by decreasing effector T cells and altering humoral responses. Immunology. 2014; 143:396–405. PMID: 24850614.

48. Eller K, Wolf D, Huber JM, Metz M, Mayer G, McKenzie AN, et al. IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J Immunol. 2011; 186:83–91. PMID: 21115728.

Fig. 1
Schematic representation of the experimental AR and treatment protocol. AR, allergic rhinitis; OVA, ovalbumin; Alum, aluminum hydroxide; i.p., intraperitoneal administration; i.n., intranasal administration.
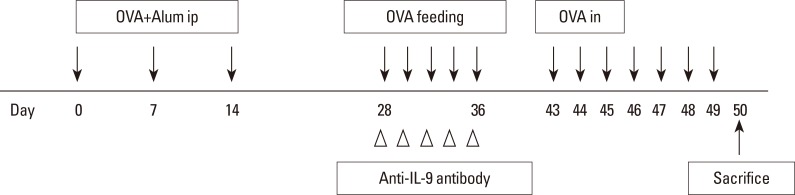
Fig. 2
Nasal symptom scores. The number of sneezes (A) and rubbing motions (B). Error bars represent standard deviations (SD). *P<0.05 vs the control group; †P<0.05 vs the Der f group.
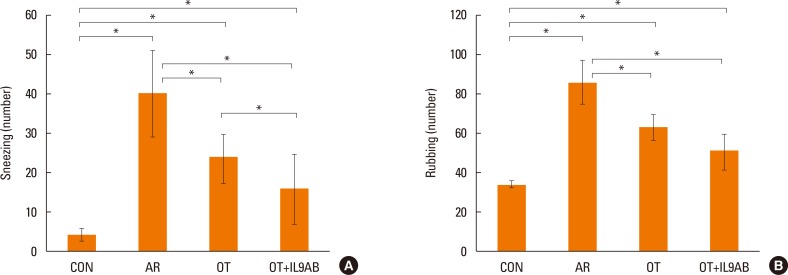
Fig. 3
Decreased OVA-specific IgE synthesis and eosinophil infiltration in nasal mucosa by the administration of anti-IL-9 antibody during induction of OT. (A) OVA-specific IgE in serum. (B) Eosinophil counts in the nasal mucosa of each study group. (C) Infiltration of eosinophils (arrows) in the nasal mucosa of BALB/c mice. (a) Control, (b) AR, (c) OT, and (d) OT+IL9AB groups. H&E stain; original magnification, ×400 (scale bar=10 µm). Error bars represent standard deviations. *P<0.05. OVA, ovalbumin; IgE, immunoglobulin E; IL, interleukin; OT, oral tolerance; H&E, hematoxylin and eosin.
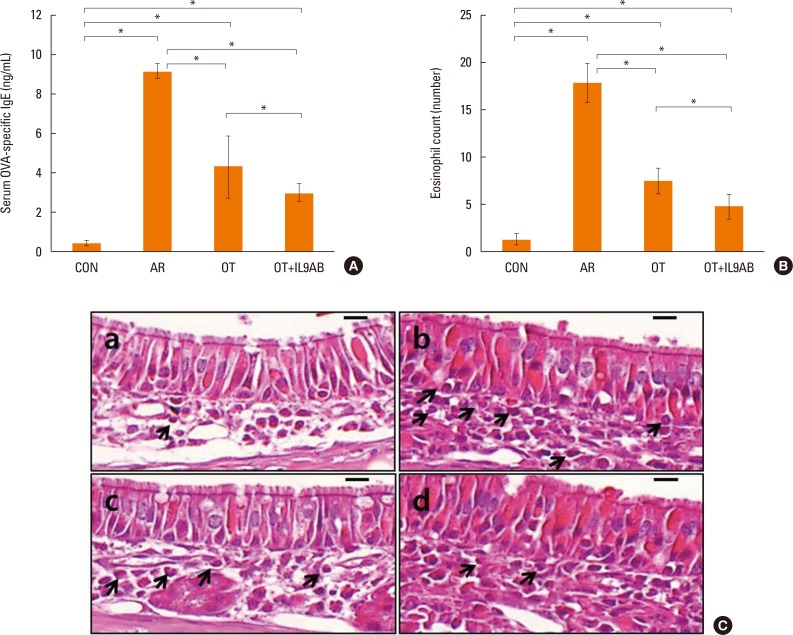
Fig. 4
Administration of anti-IL-9 antibody inhibits Th2 responses rather than Th1 responses. Quantitative analysis of IFN-γ (A), T-bet (B), IL-4 (C), and GATA3 (D) mRNA expression in nasal mucosa by real-time PCR. Error bars represent SD. *P<0.05. Th, T-helper; IFN, interferon; PCR, polymerase chain reaction; SD, standard deviation; other abbreviation as Fig. 3.
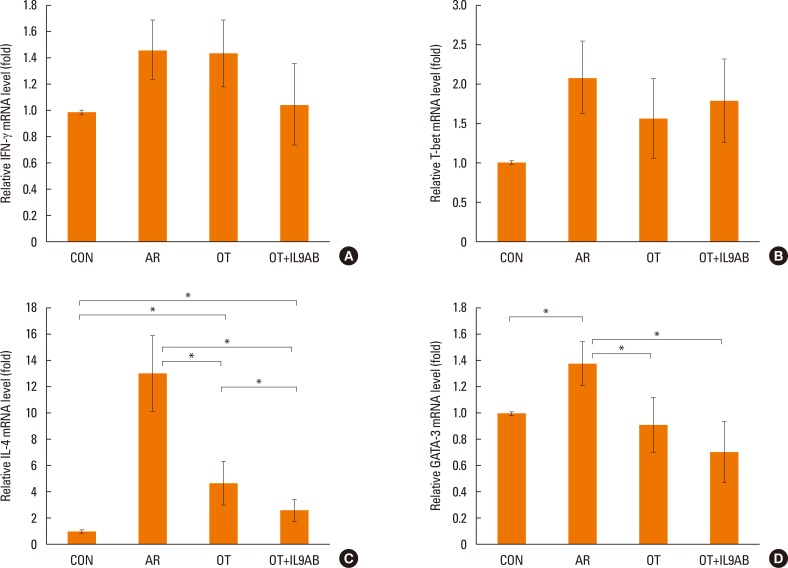
Fig. 6
Inhibition of mRNA expression of PU.1 and ROR-γt by the administration of anti-IL-9 antibody. Quantitative analysis of PU.1 (A) ROR-γt (B), and IL-17 (C) mRNA expression in nasal mucosa by real-time PCR. Error bars represent SD. Abbreviations as in Fig. 4. *P<0.05.
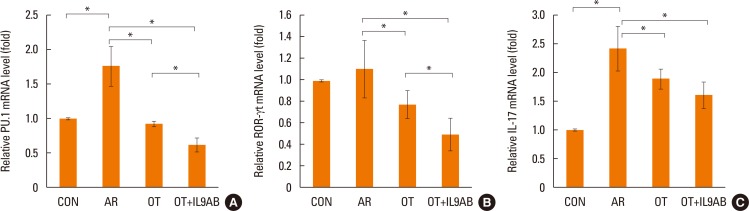
Fig. 7
Increased Foxp3, IL-10, and TGF-β mRNA expression and Foxp3 protein level by the administration of anti-IL-9 antibody. Quantitative analysis of Foxp3 (A), IL-10 (B), and TGF-β (C) mRNA expression in nasal mucosa by real-time PCR. Error bars represent SD. *P<0.05. TGF, transforming growth factor; other abbreviation as Fig. 4.
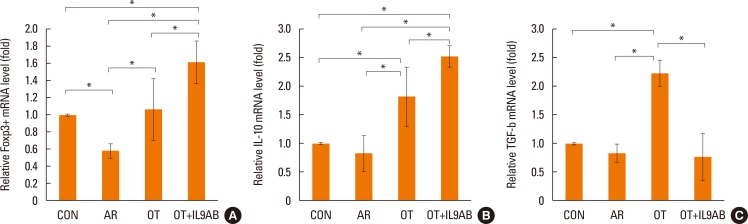
Fig. 8
Increased induction of CD4+CD25+Foxp3+ T cells by the administration of anti-IL-9 antibody. Flow cytometric analysis of CD4+CD25+Foxp3+ T cell subsets. (A) Representative fluorescence-activated cell sorting analysis of each group. The upper right quadrant represents CD4+CD25+Foxp3+ T cells. (B) The percentages of CD4+CD25+Foxp3+ T cells as a proportion of total splenic mononuclear cells. Error bars represent SD. Abbreviations as in Fig. 4. *P<0.05.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download