Abstract
Purpose
CpG oligodeoxynucleotide (CpG-ODN), a TLR9 agonist, activates innate immunity and induces Th1 response. Although the immune modulatory effect of CpG-ODN has been extensively studied, its function in cockroach extract-induced allergic asthma has not been studied. Here, we investigated the inhibitory function of CpG-ODN in cockroach extract-induced asthma in mice with different treatment schemes.
Methods
Scheme 1: BALB/C mice were intra-nasally co-administered by cockroach extract and CpG-ODN twice a week for 3 weeks; Scheme 2: The mice were intra-nasally pre-treated with CpG-ODN at day 0 and cockroach allergen challenge was performed from day 3 as in scheme 1. Scheme 3: Cockroach allergen challenge was performed as in scheme 1 and CpG-ODN was post-treated at day 21. Then, BAL cell count, flow cytometric analysis of alveolar macrophages, regulatory T cells, and lung tissue histology, Th1 and Th2 cytokines, serum IgE, cockroach specific IgE, IgG1/IgG2a ratio, and airway hyper-responsiveness were evaluated.
Results
Mice with repeated intra-nasal exposure to CpG-ODN showed a dramatic decrease in eosinophilic inflammation, goblet cell hyperplasia, and airway hyper-responsiveness with reduction of IL-13, IL-5, and serum IgE, cockroach specific IgE and IgG1/IgG2a ratio. This inhibitory function might be related to the up-regulation of IL-10 and CD4+Foxp3+ regulatory T cells in the lung. Interestingly, one-time challenge of CpG-ODN either prior or posterior to cockroach extract exposure could modulate airway inflammation and hyper-responsiveness via increase of Th1 response.
Asthma and allergies are the most common chronic inflammatory diseases associated with cockroach or house dust mite (HDM) allergens characterized by airflow obstruction, airway eosinophilic inflammation, goblet cell metaplasia, airway remodeling, and airway hyper-responsiveness (AHR).123 Th2 cytokines such as IL-4, IL-5, IL-9, and IL-13 derived from helper T cells and other cell types play a critical role in the pathophysiology of asthma,4 and the balance between Th1 and Th2 cells is thought to be important in controlling disease symptoms.5 The suppression of Th2 responses by induction of Th1 response or regulatory T cells or by inhibiting Th2 inducing factors such as IL-4, IL-5, and IL-13 have been extensively investigated for the development of therapeutic drugs for asthma.6
CpG oligodeoxynucleotide (CpG-ODN) is a synthetic TLR9 ligand, consisting of an unmethylated dinucleotide that activates immune responses similar to those in response to bacterial DNA.78 CpG-ODN treatment has been extensively studied to stimulate both innate and adaptive immunity as it induces TNF-α expression in macrophages,9 augments IFN-γ and IL-12 expression and enhances the cytotoxic activity of NK cells1011 and Th1 response, which can be used as immune adjuvants and to modulate Th2 responses.1213 In addition, CpG-ODN has been shown to enhance type I IFN production from plasmacytoid dendritic cells (pDCs),1415 and induce IL-10 producing regulatory T cells by indoleamine 2,3-dioxygenase (IDO) 1 production.1617 Thus, CpG-ODN has been investigated as a potent preventive as well as a therapeutic immune modulator in allergic inflammatory diseases.1819
Cockroaches are known to be a significant environmental allergen resulting in atopic asthma,2021 and among the various environmental factors, cockroach allergen is recognized as a major risk factor for pathogenesis of allergic asthma in humans.222324 Children who are exposed to high bedroom levels of cockroach allergens showed an increased incidence of allergic asthma, compared to other children.2526 Also, among the many indoor allergens, only cockroaches were significantly related to recurrent asthmatic wheezing.23 Previous preventive and therapeutic CpG-ODN treatment has been shown to suppress eosinophilic inflammation and hyper-responsiveness in the OVA/Alum-induced asthma model.27 More recently, CpG-ODN treatment also suppressed airway inflammation and hyper-responsiveness in the HDM-induced asthma model.28
However, the immune modulatory effects of CpG-ODN in cockroach-induced asthma have not been investigated. The aim of the present study is to investigate the immune modulatory effect of intra-nasally administered CpG-ODN in a cockroach-induced asthma model with different treatment schemes.
Female BALB/C mice, 6 to 8 weeks old, were purchased from Orient Bio (Daejeon, Korea). All mice were housed under specific pathogen-free conditions, and maintained on a 12 hours light-dark cycle with regular chow and autoclaved water ad libitum. All experiments described in this study were approved by the Animal Research Ethics Board of Yonsei University.
Crude cockroach extract was prepared as previously described.29 Briefly, 30 g of live or frozen Blattela germanica were pulverized in liquid nitrogen. The sample was then defatted in 200 mL of 1:1 ethyl ether/ethyl acetate and extracted overnight with slow stirring at 4℃ in phosphate-buffered saline (pH 7.4) containing 6 mM β-mercaptoethanol and 1 mg/mL 1-phenyl-3-(2-thiazolyl)-2-thiourea to prevent melanization. The extract was then centrifuged at 8,000 rpm for 30 minutes at 4℃, and the supernatant was filtered through a 0.22 mm filter and then lyophilized.
The CpG-ODN and control ODNs were used as previously described.11 The CpG-ODNs contain two CpG (1826) motifs (5´-TCC ATG ACG TTC CTG ACG TT-3´). The control ODNs had equivalent lengths without functional CpG motifs (5´-TCG ATG AGC TTC CTG AGT CT-3´). The ODNs were synthesized and purified using a HPLC by Genotech, Inc. (Daejon, Korea).
For the co-treatment scheme, 6- to 8-week old BALB/C mice were intra-nasally co-sensitized by 120 µg cockroach extract and 3 µg of control ODN or CpG-ODN, twice a week for 3 weeks. For the pre-treatment scheme, mice were administered 3 µg of the control ODN or of CpG-ODN intra-nasally once on day 0, and then 120 µg intra-nasal cockroach allergen challenge was performed twice a week for 3 weeks. Four days after the last challenge, the mice were sacrificed and pathological changes of the lungs were examined. For the post-treatment scheme, mice were sensitized by intra-nasal administration of 120 µg cockroach extract twice a week for 3 weeks. Two days after the last challenge, 3 µg of control ODN or CpG-ODN was intra-nasally administrated, and then the mice were sacrificed and analyzed at 2 days later of CpG-ODN treatment.
Four days after the last challenge, airway hyper-responsiveness (AHR) was measured with a flexiVent 5.1® small animal ventilator (SCIREQ, PQ, Canada). In detail, mice were challenged with a saline control aerosol followed by increasing concentrations of methacholine (MeCh) (Sigma-Aldrich, MO, USA; 3.1, 6.25, 12.5, 25, and 50 mg/mL). Aerosols were generated with an ultrasonic nebulizer and delivered to the inspiratory line of the flexiVent using a bias flow of medical air. Each aerosol was delivered for 10 seconds during which time regular ventilation was maintained. Two measurements were made at 1-minute intervals following each aerosol.30
To collect bronchoalveolar lavage (BAL) fluid, the lungs were lavaged with 1 mL of Hank's balanced salt solution through an intubation tube. Total cell numbers were counted with a hemocytometer. BAL fluid was centrifuged at 1,500 rpm for 3 minutes at 4℃, and then smears of BAL cells were prepared by cytocentrifugation (Thermo, MA, USA) at 1,000 rpm for 3 minutes. All smears were stained with a Hemacolor staining kit (Merck, Darmstadt, Germany). Differential cell counts in BAL cells were done for at least 200 leukocytes, using standard hemocytologic procedures to classify macrophages, neutrophils, eosinophils, and lymphocytes. After collecting BAL fluid, one lung was removed and homogenized in 3 mL of lysis buffer T-PER@ tissue protein extraction reagent (Thermo, MA, USA) using a tissue homogenizer (Biospec Products, OK, USA). Homogenates were incubated at 4℃ for 30 minutes, then centrifuged at 2,500 rpm for 10 minutes. Supernatants were collected, passed through a 0.45 µm filter (Gelman Sciences, MI, USA), and then stored at -70℃ for assessment of cytokine levels.
All cytokine ELISA kits were purchased from R&D Systems (MN, USA). IL-5, IL-13, IFN-γ, and IL-10 in the lung tissue homogenate were analyzed by ELISA following the manufacturer's protocols. Total IgE levels in mice sera were measured using the mouse IgE ELISA Set (BD bioscience, CA, USA). Cockroach specific IgE, IgG1, and IgG2a in serum were analyzed by sandwich ELISA. Briefly, 0.1 mg/mL of cockroach allergen was coated on 96-well plate for overnight at 4℃, then the plate was washed and applied with blocking buffer for 1 hour. 10X diluted samples were incubated for overnight at 4℃. To measure cockroach specific IgE level, biotin conjugated anti-mouse IgE antibody and Streptavidin-HRP (Biolegend, CA, USA) was further applied for visualize the signal. HRP conjugated anti-mouse IgG1 and IgG2a antibodies (BD bioscience, CA, USA) were used for measure cockroach specific IgG1 and IgG2a.
After BAL fluid collection, the left lung was inflated with 10% formalin at a standard pressure. Lung tissues were then embedded in paraffin, and 3-µm-thick sections were cut and stained with periodic acid-Schiff (PAS) to determine goblet cell hyperplasia. Stained tissue sections were examined with an Olympus BX40 microscope in conjunction with an Olympus U-TV0.63XC digital camera (Olympus Corp., NY, USA). Images were acquired using DP Controller and Manager software (Olympus Corp., NY, USA). The number of PAS-positive cells per millimeter of bronchial basement membrane (mmBM) was measured by MetaMorph 4.6 (Universal Imaging, PA, USA).
To collect white blood cells from the lung, the lung tissue was isolated and minced into small pieces, mechanically disintegrated, filtered through 70 µm cell strainer, and then RBCs were lysed. To determine alveolar cell, interstitial macrophage, and monocyte populations, the collected cells were stained with fluorescent conjugated antibodies including anti-mouse CD45-PerCP-Cy5.5, CD11c-APC, and F4/80-PE antibodies (Biolegend, CA, USA) for 30 minutes and analyzed by flow cytometry. To evaluate the regulatory T cell population, the cells were stained with anti-mouse CD4-PerCP-Cy5.5 antibody (Biolegend, CA, USA), further fixed, permeabilized and stained with anti-mouse Foxp3-PE antibody (Biolegend, CA, USA) and then analyzed by flow cytometry.
To determine the immune modulatory effects of CpG-ODN treatment in cockroach-induced allergic asthma, 3 µg of CpG-ODN or control ODN was intra-nasally co-treated with 120 µg of cockroach extract twice a week for 3 weeks (Fig. 1A). Repeated cockroach allergen challenge with control ODN significantly increased airway resistance to MeCh while 6 times CpG-ODN co-treatment significantly inhibited AHR (Fig. 1B). Also, repeated cockroach allergen challenge with control ODN significantly increased the number of inflammatory cells in the BAL fluid including macrophages, neutrophils, and eosinophils, while CpG-ODN treatment dramatically reduced the number of inflammatory cells in the BALF (Fig. 1C and D). In addition, PAS-stained histological analysis of the lung tissue suggested that goblet cell hyperplasia, hypertrophy in the epithelium, mucus production, and infiltration of inflammatory cells was increased in repeated cockroach allergen challenge in the control ODN treated group, while the CpG-ODN treatment remarkably reduced the number of goblet cells and infiltrated inflammatory cells (Fig. 1E and F). Collectively, repeated intra-nasal co-treatment of CpG-ODN strongly ameliorated airway inflammation and hyper-responsiveness induced by cockroach allergen challenge.
Alveolar macrophages which derived from circulating blood monocytes are the most abundant pulmonary macrophage, and serve as a first line of defense against foreign invaders of the lung tissue.31 The low ratio of alveolar to interstitial macrophages is a hallmark of lung inflammation.32 We isolated mononuclear cells from the lung tissue and the pulmonary macrophage population was analyzed by flow cytometry. The F4/80+CD11chigh alveolar macrophage proportion was dramatically reduced in the cockroach extract and control ODN-treated group, while they had a significantly increased population of F4/80+CD11cint interstitial macrophages. CpG-ODN treatment dramatically reversed the alveolar and interstitial macrophage population (Fig. 2A-C). In addition, F4/80+CD11clow monocytes were also significantly reduced by repeated CpG-ODN treatments (Fig. 2D), collectively suggesting that CpG-ODN contributes to immune tolerance in order to maintain the homeostasis of pulmonary inflammation and macrophage populations.
To understand the mechanism of ameliorated allergic inflammation and hyper-responsiveness in mice related to CpG-ODN treatment, we analyzed serum IgE levels, inflammatory cytokines and immune-suppressive regulatory T cell populations in the lungs. Serum IgE levels from the cockroach-extract and control ODN-treated group were significantly increased compared to the Sham group while repeated CpG-ODN treatment significantly inhibited IgE production, suggesting that antibody production against this allergen by plasma cells was significantly abrogated by the CpG-ODN challenge (Fig. 3A). Also, the level of cockroach-specific IgE and IgG1/IgG2a ratio were significantly down-regulated by CpG-ODN treatment (Fig. 3B and C). In addition, the levels of Th2 cytokines such as IL-13 (Fig. 3D), IL-5 (Fig. 3E) in the lung were dramatically reduced by repeated CpG-ODN treatment. Moreover, IFN-γ production was also inhibited by CpG-ODN treatment (Fig. 3F) suggesting its potency in immune modulation. Importantly, the level of IL-10 was increased by CpG-ODN treatment (Fig. 3G) with a significantly enhanced proportion of Foxp3+ regulatory CD4 T cells (Fig. 3H), suggesting that repeated CpG-ODN challenge induces regulatory T cells in the lung to prevent or inhibit allergic airway inflammation.
Since DNA vaccinations, such as the Bacillus Calmette-Guerin (BCG) vaccine, were demonstrated to play preventive roles in allergic inflammation in the lung3334 and CpG-ODN is known to mediate the equivalent TLR9 signaling pathway to stimulate antigen presenting cells,35 we attempted a CpG-ODN vaccination scheme to determine whether it could modulate the immune system and prevent Th2-mediated inflammatory diseases via induction of Th1 cells prior to cockroach allergen challenge. CpG-ODN was intra-nasally administrated on day 0, and then intra-nasal cockroach extract challenge was performed 6 times over 3 weeks (Fig. 4A). One-time CpG treatment prior to cockroach allergen challenge prevented airway hyper-responsiveness while the control ODN-treated group showed increased airway resistance to MeCh (Fig. 4B). The number of BAL cells including lymphocytes, eosinophils, and neutrophils was significantly reduced in CpG-ODN-treated mice (Fig. 4C and D). On histopathological analysis, increased goblet cell hyperplasia, airway inflammation with infiltrated mononuclear cells, and increased airway epithelium thickness were dramatically prevented by one time CpG-ODN pre-treatment (Fig. 4E and F). Collectively, these data suggest that a CpG-ODN challenge prior to allergen exposure has a remarkable preventive effect in the cockroach allergen-induced asthma model.
To determine the prevention mechanism of one-time challenge of CpG-ODN, we analyzed the total IgE and cockroach allergen specific antibody levels in the serum, Th1 and Th2 cytokine levels and the proportion of regulatory T cells in the lung. Total and cockroach specific IgE serum levels were significantly reduced by CpG-ODN pre-treatment compared to the control ODN-treated group (Fig. 5A and B). Also, cockroach specific IgG1/IgG2a ratio was significantly down-regulated by one-time CpG-ODN pre-treatment (Fig. 5C). In addition, the level of IL-13 in the lung decreased (Fig. 5D). More dramatically, the IL-5 level which is important for eosinophilic inflammation was comparable to that of the sham group suggesting that one-time CpG treatment strongly prevented Th2 inflammation (Fig. 5E). CpG-ODN pre-treatment showed increased IFN-γ expression in the lung (Fig. 5F), while IL-10 (Fig. 5G) and Foxp3+ regulatory CD4 T cells (Fig. 5H) were not significantly affected, suggesting that intra-nasal CpG-ODN challenge prior to cockroach allergen exposure stimulates Th1 immune responses in the lung which inhibits the development of Th2 inflammation to the cockroach allergen.
Because CpG-ODN has been studied to rapidly stimulate innate immune system to induce Th1 responses, we hypothesized that it might have a therapeutic effect in allergic asthma model. To determine the therapeutic effects of CpG-ODN, 3 µg of CpG-ODN or control ODN was intra-nasally treated at day-21 and 120 µg of cockroach extract was administered twice a week for 3 weeks (Fig. 6A). The therapeutic treatment of CpG-ODN posterior to cockroach allergen challenge could modulate AHR while the control ODN-treated group showed increased airway resistance to MeCh (Fig. 6B). The number of BAL cells including macrophage, eosinophils, and neutrophils was significantly reduced in CpG-ODN-treated mice (Fig. 6C and D). Also, increased goblet cell hyperplasia, airway inflammation with infiltrated mononuclear cells, and increased airway epithelium thickness were significantly recovered by one-time CpG-ODN post-treatment (Fig. 6E and F). Collectively, these data suggest that there is significant therapeutic effect of CpG-ODN in cockroach allergen-induced asthma model.
To determine the therapeutic mechanism of one-time challenge of CpG-ODN, we analyzed the serum total IgE and cockroach specific antibody levels, Th1 and Th2 cytokine levels and the proportion of regulatory T cells in CpG-ODN post-treated mouse model. Interestingly, CpG-ODN post-treatment significantly reduced total IgE and cockroach specific IgE serum levels compared to the control ODN-treated group (Fig. 7A and B). Also, the cockroach specific IgG1/IgG2a ratio was down-regulated by CpG-ODN post-treatment (Fig. 7C). In addition, the levels of IL-13 (Fig. 7D) and IL-5 (Fig. 7E) in the lung decreased compare to control-ODN group, suggesting that one-time CpG post-treatment could modulate Th2 inflammation. Interestingly, CpG-ODN post-treatment showed significantly increased IFN-γ expression in the lung (Fig. 7F), while IL-10 (Fig. 7G) and Foxp3+ regulatory T cells (Fig. 7H) were comparable to control-ODN group, suggesting that intra-nasal CpG-ODN challenge posterior to cockroach allergen exposure stimulates Th1 immune responses in the lung as like CpG pre-treatment, and modulates Th2 inflammation in a cockroach-induced asthma model.
In the present study, we demonstrate the immune modulatory effect of CpG-ODN in a cockroach-induced asthma model by three different treatment schemes. In any scheme of treatment such as co-, pre-, and post-treatment, intra-nasal CpG-ODN administration dramatically inhibits eosinophilic airway inflammation, goblet cell hyperplasia, airway remodeling, and hyper-responsiveness, which are typical pathophysiologic changes in human asthma. However, the mechanism of action of CpG-ODN is different based on the treatment schemes. Repeated co-treatment of CpG-ODN and cockroach allergen increased IL-10 and Foxp3+ regulatory T cells in the lung, which could ameliorate Th2 responses, while one-time treatment of CpG-ODN, either prior or posterior to cockroach allergen challenge, enhances Th1 response, which could prevent or modulate Th2 response in the lung. These results emphasize the advantage of usage of CpG-ODN, a TLR9 ligand, to modulate allergic Th2 responses and suggest different mode of action of CpG-ODN based on the treatment scheme.
Historically, Tokunaga et al.36 first reported that bacterial DNA induces anti-tumor activity. Repeated injections of nucleotide fractions, extracted from the Mycobacterium bovis strain BCG, prevent metastasis in animal models, and DNase treatment reduces the anti-tumor effects of nucleotide fractions, suggesting that bacterial DNA induces anti-tumor activity.36 Also, bacterial DNA has been found to induce IFN production and elevated NK cell activity, which suppresses tumorigenesis.37 In 1995, Krieg et al.8 reported that a specific DNA motif, which consists of unmethylated CpG, induces B cell proliferation and Th1 immune response.
CpG-ODN is a synthetic TLR9 ligand that activates TLR9-expressing pDC and B cells to increase co-stimulatory molecules, chemokines such as CCR7, and to produce cytokines including IFN-α.738 When CpG-ODN is internalized into an endosomal compartment, it can be recognized by TLR9 and consequently activates the MyD88/TFAF6 pathway to induce type I IFN.39 Also, CpG-ODN induces expression of pro-inflammatory cytokines and Th1-related cytokines.10 Among various cytokines, IFN-γ is suggested to be the most critical cytokine, and is involved in the amelioration of Th2 inflammation.40 The pre-administration of CpG-ODN 48 hours before ragweed allergen challenge prevented allergic asthma through up-regulation of IFN-γ expression. CpG-ODN could not prevent the pathogenesis of allergic asthma in IFN-γ knock out (KO) mice, suggesting that IFN-γ is a critical factor for the immune modulatory function of CpG-ODN.40 However, repeated CpG-ODN treatment partially ameliorated eosinophilic inflammation and Th2 cytokine production in IFN-γ and IL-12 KO mice, suggesting that there could be alternative mechanisms.41 Recently, pDCs activated by CpG-ODN were shown to be involved in the generation of CD4+CD25+ regulatory T cells and to induce IDO expression under inflammatory conditions.1617 In addition, CpG-ODN demonstrated anti-inflammatory function in pDC in a PD-1/PD-L1 dependent manner.42 More recently, increased Foxp3 mRNA levels were measured in CD4 T cells with high doses of CpG-ODN treatment. CpG-ODN treatment reduced eosinophilia and IgE production in an A. fumigatus-induced allergic inflammation model with increases in Foxp3+ CD4 T cells from thoracic lymph nodes.43 Given the relevant role of CpG-ODN in Th1 and regulatory T cell generation, in the present study, CpG-ODN could modulate Th2 responses in cockroach-induced asthma by induction of Th1 or regulatory T cells in the lung based on the treatment schemes.
Based on its immune modulatory functions, CpG-ODN has previously been broadly applied to various animal models. It has been shown to improve the efficacy of the heat-killed Leishmania vaccine in Rhesus macaques and to have a protective function in simian immunodeficiency virus-infected Rhesus macaques with a 1,000-fold reduced heat-killed parasite load.4445 In an anthrax infection model, CpG-ODN combined with an anthrax vaccine adsorbed significantly up-regulate antibody function and survival rates of infected animals.46 In addition, CpG-ODN treatment ameliorated murine cervical carcinoma and improved survival rates due to its anti-tumor activity.47 Collectively, many studies have focused on the function of CpG-ODN as an effective agent for inducing Th1 immune responses as a vaccine adjuvant or anti-tumor agent. In addition, CpG-ODN treatment was previously attempted in asthma models.2740 The systemic administration of CpG-ODN recovered established eosinophilic inflammation in an OVA-induced asthma model.27 CpG-ODN also prevented pollen allergen-induced asthma through up-regulation of IL-10 and IFN-γ.48 More recently, treatment of CpG-ODN with HDM allergen clearly inhibited airway hyper-responsiveness, goblet cell hyperplasia, and airway inflammation.28 Interestingly, low-dose CpG-ODN treatment induced Th1 immune responses, while high dose CpG-ODN increased regulatory functions such as increased regulatory T cell populations and IL-10 cytokine production.4049 Our results also demonstrated that repeated treatment of CpG-ODN induced Foxp3+ regulatory CD4 T cells and IL-10 production in the lung, while either one-time pre- or post-treatment of CpG-ODN up-regulated Th1 immune response, suggesting that the consequences of TLR9 signaling differ based on the quantity of the CpG-ODN challenge.
Based on accumulated studies on CpG-ODN in various disease models, many clinical trials have investigated the use of CpG-ODN as an immune modulatory agent.5051 CpG-ODN has not shown significant toxicity, and its side effects are reported to be mild and do not interfere with daily life.52 Companies such as Pfizer and GlaxoSmithKline have developed CpG-ODN as a vaccine adjuvant for various types of tumors.53 Although CpG-ODN has been extensively investigated for tumor therapy, clinical trials for allergic asthma have not yet been conducted.
Cockroach allergen exposure is a critical risk factor for allergic asthma severity and frequency in humans.232526 In the present study, we demonstrate that CpG-ODN can modulate cockroach-induced allergic airway inflammation and Th2 responses by induction of Th1 cells or regulatory T cells in the lung. Collectively, administration of CpG-ODN or a TLR9 agonist treatment, as a DNA vaccine or even as a therapeutic agent, could be advantageous in preventing and modulating allergic inflammation, particularly in asthma.
Figures and Tables
Fig. 1
Repeated intra-nasal CpG-ODN co-treatment ameliorates cockroach-induced airway inflammation and hyper-responsiveness. The experimental scheme of CpG-ODN co-treatment in the cockroach-induced asthma model (A). Airway hyper-responsiveness was measured by a flexiVent 5.1® small animal ventilator (B). Total BAL cells were harvested, and then cells were stained by a Hemacolor staining kit and analyzed by microscope (C). The number of macrophages, lymphocytes, eosinophils and neutrophils in BAL fluid were counted (D). The paraffin-embedded lung tissue was prepared as a slide for PAS staining for tissue histology (E), and PAS-positive cells were counted in the indicated area (F). The values are presented as the mean±SD of the results from 5 mice per group. *P<0.05; **P<0.01. CR, cockroach.
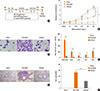
Fig. 2
Repeated intra-nasal CpG-ODN co-treatment changes the pulmonary macrophage population. Total lung cells from each group of mice were isolated and stained with anti-mouse CD45-PerCP-Cy5.5, CD11c-APC, F4/80-PE antibodies (A). The percentages of CD45+F4/80+CD11chigh alveolar macrophage (B), CD45+F4/80+CD11cint interstitial macrophage (C), and CD45+F4/80+CD11clow monocyte (D) were analyzed. Data are presented as the mean±SD of the results from 5 mice per group. *P<0.05. CR, cockroach.

Fig. 3
Repeated intra-nasal CpG-ODN co-treatment reduces Th2 inflammation by induction of Foxp3+ regulatory T cells in the lung. Total IgE (A), cockroach specific IgE (B), and Ig1/IgG2a ratio (C) in the serum of each group was measured by ELISA. The level of IL-13 (D), IL-5 (E), IFN-γ (F), and IL-10 (G) in the lung lysate from each group of mice was measured by ELISA. The percentage of CD4+Foxp3+ regulatory T cells in the lung was analyzed by flow cytometry (H). Data are presented as the mean±SD of the results from 5 mice per group. *P<0.05; **P<0.01. CR, cockroach.

Fig. 4
Intra-nasal CpG-ODN treatment prior to cockroach allergen challenge prevents allergic airway inflammation and hyper-responsiveness. The experimental scheme of CpG-ODN pre-treatment in the cockroach-induced asthma model (A). Airway hyper-responsiveness was measured by a flexiVent 5.1® small animal ventilator (B). Total BAL cells were harvested, then a Hemacolor staining kit was used to stain the cells which were analyzed by microscope (C). The number of macrophages, lymphocytes, eosinophils, and neutrophils from BAL fluid were counted (D). Paraffin-embedded lung tissue was prepared as a slide for PAS staining for tissue histology (E), and PAS-positive cells were counted in the indicated area (F). The values are presented as the mean±SD of the results from 5 mice per group. *P<0.05; **P<0.01. CR, cockroach.
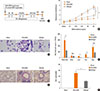
Fig. 5
Intra-nasal CpG-ODN treatment prior to cockroach allergen challenge prevents Th2 inflammation by induction of Th1 response in the lung. Total IgE (A), cockroach specific IgE (B), and IgG1/IgG2a ratio (C) in the serum of each group was measured by ELISA. Also, IL-13 (D), IL-5 (E), IFN-γ (F), and IL-10 (G) levels in the lung lysate from each group of mice were measured by ELISA. The percentage of CD4+Foxp3+ regulatory T cells in the lung was analyzed by flow cytometry (H). Data are presented as the mean±SD of the results from 5 mice per group. *P<0.05; **P<0.01. CR, cockroach.

Fig. 6
Intra-nasal CpG-ODN treatment posterior to cockroach allergen challenge modulates allergic airway inflammation and hyper-responsiveness. Experimental scheme of CpG-ODN post-treatment to cockroach-induced asthma model (A). Airway hyper-responsiveness was measured by flexiVent 5.1® small animal ventilator (B). Total BAL cells were harvested, then a Hemacolor staining kit was used to stain the cells which were analyzed by microscope (C). The number of macrophages, lymphocytes, eosinophils, and neutrophils from BAL fluid were counted (D). Paraffin-embedded lung tissue was prepared as a slide for PAS staining for tissue histology (E), and PAS-positive cells were counted in the indicated area (F). The values are presented as the mean±SD of the results from 5 mice per group. *P<0.05; **P<0.01. CR, cockroach.
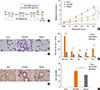
Fig. 7
Intra-nasal CpG-ODN treatment posterior to cockroach allergen challenge modulates Th2 inflammation by increase of Th1 response in the lung. The total IgE (A), cockroach specific IgE (B), and IgG1/IgG2a ratio in the serum (C) were measured by ELISA. IL-13 (D), IL-5 (E), IFN-γ (F), and IL-10 (G) in lung lysate from each group of mice were measured by ELISA. The percentage of CD4+Foxp3+ regulatory T cells in the lung was analyzed by flow cytometry (H). Data are presented as the mean±SD of the results from 5 mice per group. *P<0.05; **P<0.01. CR, cockroach.

ACKNOWLEDGMENTS
This study was supported by grants from the Basic Science Research Program of the National Research Foundation of Korea (NRF-2013R1A1A2A10060048), the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and the Ministry of Health & Welfare, Republic of Korea (HI14C0234), and Hanyang University (HY-2011-00000001004) to J.M. Choi.
References
1. Djukanović R, Roche WR, Wilson JW, Beasley CR, Twentyman OP, Howarth RH, et al. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990; 142:434–457.
2. Jeong KY, Son M, Lee JH, Hong CS, Park JW. Allergenic characterization of a novel allergen, homologous to chymotrypsin, from german cockroach. Allergy Asthma Immunol Res. 2015; 7:283–289.
3. Park HJ, Lee JH, Park KH, Ann HW, Jin MN, Choi SY, et al. A nationwide survey of inhalant allergens sensitization and levels of indoor major allergens in Korea. Allergy Asthma Immunol Res. 2014; 6:222–227.
4. Robinson D, Hamid Q, Bentley A, Ying S, Kay AB, Durham SR. Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J Allergy Clin Immunol. 1993; 92:313–324.
5. Ray A, Cohn L. Altering the Th1/Th2 balance as a therapeutic strategy in asthmatic diseases. Curr Opin Investig Drugs. 2000; 1:442–448.
6. Barnes PJ. New drugs for asthma. Discov Med. 2004; 4:421–426.
7. Krieg AM, Matson S, Fisher E. Oligodeoxynucleotide modifications determine the magnitude of B cell stimulation by CpG motifs. Antisense Nucleic Acid Drug Dev. 1996; 6:133–139.
8. Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995; 374:546–549.
9. Schwartz DA, Quinn TJ, Thorne PS, Sayeed S, Yi AK, Krieg AM. CpG motifs in bacterial DNA cause inflammation in the lower respiratory tract. J Clin Invest. 1997; 100:68–73.
10. Chace JH, Hooker NA, Mildenstein KL, Krieg AM, Cowdery JS. Bacterial DNA-induced NK cell IFN-gamma production is dependent on macrophage secretion of IL-12. Clin Immunol Immunopathol. 1997; 84:185–193.
11. Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci U S A. 1996; 93:2879–2883.
12. Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997; 186:1623–1631.
13. Kline JN, Waldschmidt TJ, Businga TR, Lemish JE, Weinstock JV, Thorne PS, et al. Modulation of airway inflammation by CpG oligo-deoxynucleotides in a murine model of asthma. J Immunol. 1998; 160:2555–2559.
14. Krug A, Rothenfusser S, Hornung V, Jahrsdörfer B, Blackwell S, Ballas ZK, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001; 31:2154–2163.
15. Takauji R, Iho S, Takatsuka H, Yamamoto S, Takahashi T, Kitagawa H, et al. CpG-DNA-induced IFN-alpha production involves p38 MAPK-dependent STAT1 phosphorylation in human plasmacytoid dendritic cell precursors. J Leukoc Biol. 2002; 72:1011–1019.
16. Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004; 173:4433–4442.
17. Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009; 183:2475–2483.
18. Kline JN, Ballas ZK. DNA immunomodulation of asthma. Clin Allergy Immunol. 2002; 16:551–564.
19. Krieg AM, Kline JN. Immune effects and therapeutic applications of CpG motifs in bacterial DNA. Immunopharmacology. 2000; 48:303–305.
20. Mendoza J, Snyder RD. Cockroach sensitivity in children with bronchial asthma. Ann Allergy. 1970; 28:159–163.
21. Kang B. Study on cockroach antigen as a probable causative agent in bronchial asthma. J Allergy Clin Immunol. 1976; 58:357–365.
22. Huss K, Adkinson NF Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol. 2001; 107:48–54.
23. Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Exposure to cockroach allergen in the home is associated with incident doctor-diagnosed asthma and recurrent wheezing. J Allergy Clin Immunol. 2001; 107:41–47.
24. Stelmach I, Brzozowska A, Jerzyńska J, Podsiadłowicz-Borzecka M, Bobrowska M, Majak P, et al. Hypersensitivity to cockroach allergen among children with bronchial asthma living in the Łódź district. Pol Merkur Lekarski. 2000; 9:657–661.
25. Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997; 336:1356–1363.
26. Schulaner FA. Cockroach allergen and asthma. N Engl J Med. 1997; 337:791–792.
27. Kline JN, Kitagaki K, Businga TR, Jain VV. Treatment of established asthma in a murine model using CpG oligodeoxynucleotides. Am J Physiol Lung Cell Mol Physiol. 2002; 283:L170–L179.
28. Hirose I, Tanaka H, Takahashi G, Wakahara K, Tamari M, Sakamoto T, et al. Immunomodulatory effects of CpG oligodeoxynucleotides on house dust mite-induced airway inflammation in mice. Int Arch Allergy Immunol. 2008; 147:6–16.
29. Jeong KY, Kim CR, Park J, Han IS, Park JW, Yong TS. Identification of novel allergenic components from German cockroach fecal extract by a proteomic approach. Int Arch Allergy Immunol. 2013; 161:315–324.
30. Park JW, Taube C, Joetham A, Takeda K, Kodama T, Dakhama A, et al. Complement activation is critical to airway hyperresponsiveness after acute ozone exposure. Am J Respir Crit Care Med. 2004; 169:726–732.
31. Mathias LJ, Khong SM, Spyroglou L, Payne NL, Siatskas C, Thorburn AN, et al. Alveolar macrophages are critical for the inhibition of allergic asthma by mesenchymal stromal cells. J Immunol. 2013; 191:5914–5924.
32. Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol. 2001; 70:163–170.
33. Arnoldussen DL, Linehan M, Sheikh A. BCG vaccination and allergy: a systematic review and meta-analysisl. J Allergy Clin Immunol. 2011; 127:246–253. 253.e1–253.e21.
34. Kim YJ, Kim HJ, Kang MJ, Yu HS, Seo JH, Kim HY, et al. Bacillus Calmette-Guérin Suppresses Asthmatic Responses via CD4(+)CD25(+) Regulatory T Cells and Dendritic Cells. Allergy Asthma Immunol Res. 2014; 6:201–207.
35. Warren TL, Bhatia SK, Acosta AM, Dahle CE, Ratliff TL, Krieg AM, et al. APC stimulated by CpG oligodeoxynucleotide enhance activation of MHC class I-restricted T cells. J Immunol. 2000; 165:6244–6251.
36. Tokunaga T, Yamamoto H, Shimada S, Abe H, Fukuda T, Fujisawa Y, et al. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J Natl Cancer Inst. 1984; 72:955–962.
37. Yamamoto S, Yamamoto T, Shimada S, Kuramoto E, Yano O, Kataoka T, et al. DNA from bacteria, but not from vertebrates, induces interferons, activates natural killer cells and inhibits tumor growth. Microbiol Immunol. 1992; 36:983–997.
38. Krug A, Rothenfusser S, Selinger S, Bock C, Kerkmann M, Battiany J, et al. CpG-A oligonucleotides induce a monocyte-derived dendritic cell-like phenotype that preferentially activates CD8 T cells. J Immunol. 2003; 170:3468–3477.
39. Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003; 198:513–520.
40. Sur S, Wild JS, Choudhury BK, Sur N, Alam R, Klinman DM. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J Immunol. 1999; 162:6284–6293.
41. Kline JN, Krieg AM, Waldschmidt TJ, Ballas ZK, Jain V, Businga TR. CpG oligodeoxynucleotides do not require TH1 cytokines to prevent eosinophilic airway inflammation in a murine model of asthma. J Allergy Clin Immunol. 1999; 104:1258–1264.
42. Baban B, Chandler PR, Johnson BA 3rd, Huang L, Li M, Sharpe ML, et al. Physiologic control of IDO competence in splenic dendritic cells. J Immunol. 2011; 187:2329–2335.
43. Volpi C, Fallarino F, Pallotta MT, Bianchi R, Vacca C, Belladonna ML, et al. High doses of CpG oligodeoxynucleotides stimulate a tolerogenic TLR9-TRIF pathway. Nat Commun. 2013; 4:1852.
44. Teleshova N, Kenney J, Jones J, Marshall J, Van Nest G, Dufour J, et al. CpG-C immunostimulatory oligodeoxyribonucleotide activation of plasmacytoid dendritic cells in rhesus macaques to augment the activation of IFN-gamma-secreting simian immunodeficiency virus-specific T cells. J Immunol. 2004; 173:1647–1657.
45. Verthelyi D, Kenney RT, Seder RA, Gam AA, Friedag B, Klinman DM. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J Immunol. 2002; 168:1659–1663.
46. Klinman DM, Xie H, Ivins BE. CpG oligonucleotides improve the protective immune response induced by the licensed anthrax vaccine. Ann N Y Acad Sci. 2006; 1082:137–150.
47. Weigel BJ, Rodeberg DA, Krieg AM, Blazar BR. CpG oligodeoxynucleotides potentiate the antitumor effects of chemotherapy or tumor resection in an orthotopic murine model of rhabdomyosarcoma. Clin Cancer Res. 2003; 9:3105–3114.
48. Mousavi T, Salek Moghadam A, Falak R, Tebyanian M. Co-administration of CpG oligonucleotides and chenopodium album extract reverse IgG2a/IgG1 ratios and increase IFN-gamma and IL-10 productions in a murine model of asthma. Iran J Allergy Asthma Immunol. 2008; 7:1–6.
49. Kitagaki K, Jain VV, Businga TR, Hussain I, Kline JN. Immunomodulatory effects of CpG oligodeoxynucleotides on established th2 responses. Clin Diagn Lab Immunol. 2002; 9:1260–1269.
50. Mason KA, Hunter NR. CpG plus radiotherapy: a review of preclinical works leading to clinical trial. Front Oncol. 2012; 2:101.
51. Zent CS, Smith BJ, Ballas ZK, Wooldridge JE, Link BK, Call TG, et al. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. Leuk Lymphoma. 2012; 53:211–217.
52. Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligode-oxynucleotides as immune adjuvants. Immunol Rev. 2004; 199:201–216.
53. Krieg AM. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene. 2008; 27:161–167.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download