Abstract
Purpose
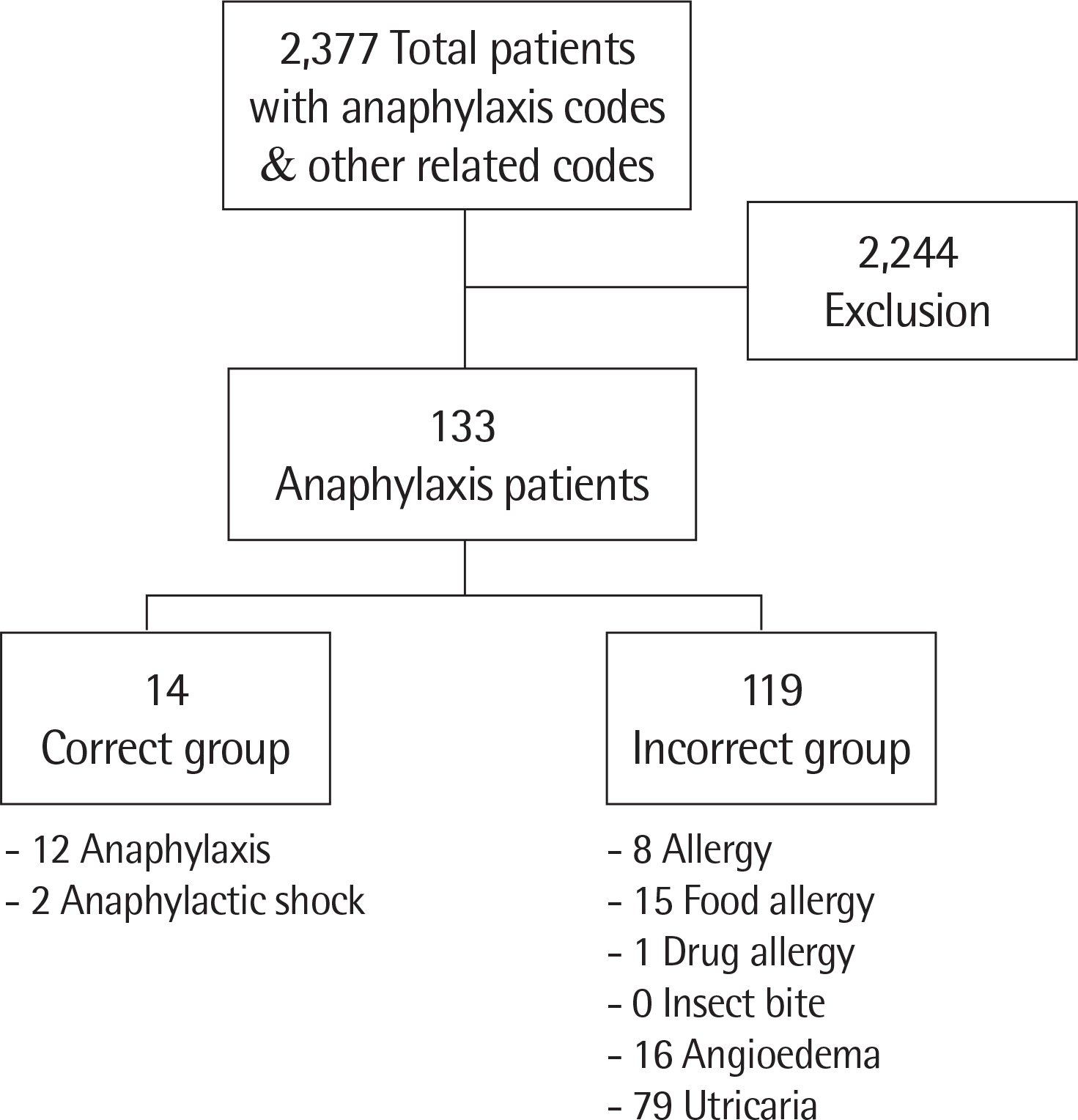
The aim of this study was to survey the accuracy of registration as anaphylaxis codes and the clinical characteristics of anaphylaxis registered correctly and incorrectly in pediatric anaphylaxis.
Methods
This study was conducted retrospectively using the medical records of patients under 15 years who visited a training hospital Emergency Department (ED) for 5 years. The study subjects were divided into the correct group (registered as anaphylaxis codes correctly) and the incorrect group (registered as other anaphylaxis related codes).
Results
Of the 133 patients, 14 belonged to the correct group and 119 to the incorrect group. The median age of the correct group was 9 years old and that of the incorrect group was 2 years old. Sex, transportation to the ED, elapsed time from exposure to ED arrival, past history of allergy, causes of anaphylaxis except drug, severity of symptom, mental status, and antihistamine use were not different between the 2 groups. Drugs as the cause of anaphylaxis and cardiovascular/neurologic symptoms were more common in the correct group. Gastrointestinal symptoms were more frequent in the incorrect group. Intravenous fluid, steroid, bronchodilator, and epinephrine were more commonly used as the treatment for anaphylaxis in the correct group. The pediatric patients treated with epinephrine tended to be registered anaphylaxis correctly.
Conclusion
More patients were registered incorrectly as other anaphylaxis-related disease codes rather than correctly as the anaphylaxis disease codes in pediatric anaphylaxis. Epinephrine use was the associated factor for being registered correctly as the anaphylaxis disease codes in pediatric anaphylaxis.
REFERENCES
1. Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004; 113:832–6.

2. Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006; 117:391–7.

3. Techapornroong M, Akrawinthawong K, Cheungpasitporn W, Ruxrung- tham K. Anaphylaxis: a ten years inpatient retrospective study. Asian Pac J Allergy Immunol. 2010; 28:262–9.
5. Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014; 133:291–307.

6. McGowan EC, Keet CA. Prevalence of self-reported food allergy in the National Health and Nutrition Examination Survey (NHANES) 2007-2010. J Allergy Clin Immunol. 2013; 132:1216–9.e5.

7. Oh JW, Pyun BY, Choung JT, Ahn KM, Kim CH, Song SW, et al. Epidemiological change of atopic dermatitis and food allergy in school-aged children in Korea between 1995 and 2000. J Korean Med Sci. 2004; 19:716–23.

8. Simons FE, Sampson HA. Anaphylaxis epidemic: fact or fiction? J Allergy Clin Immunol. 2008; 122:1166–8.

9. Dinakar C. Anaphylaxis in children: current understanding and key issues in diagnosis and treatment. Curr Allergy Asthma Rep. 2012; 12:641–9.

10. Rudders SA, Banerji A, Corel B, Clark S, Camargo CA Jr. Multicenter study of repeat epinephrine treatments for food-related anaphylaxis. Pediatrics. 2010; 125:e711–8.

11. Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004; 114:371–6.

12. Park HM, Noh JC, Park JH, Won YK, Hwang SH, Kim JY, et al. Clinical features of patients with anaphylaxis at a single hospital. Pediatr Allergy Respir Dis. 2012; 22:232–8.

13. Jin HJ. Anaphylaxis: diagnosis, management, and current barriers. Allergy Asthma Respir Dis. 2016; 4:79–81.

14. Kim WK. Diagnosis and treatment of food allergy in children. Pediatr Allergy Respir Dis. 2006; 16:274–83.
15. Nam SY. Food allergy; diagnosis and treatment. Pediatr Allergy Respir Dis. 2004; 14:119–26.
16. Braganza SC, Acworth JP, Mckinnon DR, Peake JE, Brown AF. Paediatric emergency department anaphylaxis: different patterns from adults. Arch Dis Child. 2006; 91:159–63.

17. Kelso JM. Pediatric emergency department anaphylaxis: different patterns from adults. Pediatrics. 2007; 120(Supplement 3):S121.

18. Brown SG, Blackman KE, Stenlake V, Heddle RJ. Insect sting anaphylaxis; prospective evaluation of treatment with intravenous adrenaline and volume resuscitation. Emerg Med J. 2004; 21:149–54.

19. Seo DH, Ye YM, Kim SC, Ban GY, Kim JH, Shin YS, et al. A single hospital survey of anaphylaxis awareness among health care providers and medical students. Allergy Asthma Respir Dis. 2016; 4:133–9.

20. Ye YM, Kim MK, Kang HR, Kim TB, Sohn SW, Koh YI, et al. Predictors of the severity and serious outcomes of anaphylaxis in korean adults: a multicenter retrospective case study. Allergy Asthma Immunol Res. 2015; 7:22–9.

Table 1.
International Statistical Classification of Diseases 10th Revision (ICD-10) codes related anaphylaxsis
Table 2.
General characteristics of anaphylaxis registered correctly and incorrectly
| Characteristic | Correct (n=14) | Incorrect (n=119) | P-value |
|---|---|---|---|
| Age (yr) | 9 (1.8–12.5) | 2 (0.7–9.0) | 0.025∗ |
| Age group (yr) | 0.117 | ||
| <1 | 2 (14.3) | 45 (37.8) | |
| 1–7 | 4 (28.6) | 37 (31.1) | |
| ≥7 | 8 (57.1) | 37 (31.1) | |
| Male sex | 6 (42.9) | 69 (58.0) | 0.280 |
| Transportation to ED | 0.254 | ||
| Public ambulance | 2 (14.3) | 6 (5.0) | |
| Other medical facility | 1 (7.1) | 6 (5.0) | |
| Individual transportation | 11 (78.6) | 107 (89.9) | |
| Elapsed time from (min) | |||
| Exposure to symptom onset | 10 (5–65) | 30 (5–120) | 0.402∗ |
| Symptom onset to ED arrival | 110 (57–180) | 120 (60–360) | 0.391∗ |
| Past history of allergy | 8 (57.1) | 42 (35.3) | 0.110 |
| Anaphylaxis | 1 (7.1) | 1 (0.8) | 0.200 |
| Asthma | 0 (0) | 3 (2.5) | 1.000 |
| Allergic rhinitis | 1 (7.1) | 4 (3.4) | 0.432 |
| Atopic dermatitis | 4 (28.6) | 12 (10.1) | 0.067 |
| Drug allergy | 0 (0) | 3 (2.5) | 1.000 |
| Food allergy | 3 (9.0) | 25 (13.9) | 1.000 |
| Comorbidity | 1 (7.6) | 5 (4.2) | 0.494 |
Table 3.
Causes of anaphylaxis registered correctly and incorrectly
Table 4.
Clinical characteristics of anaphylaxis registered correctly and incorrectly
| Characteristic | Correct (n=14) | Incorrect (n=119) | P-value |
|---|---|---|---|
| Symptoms | |||
| Cutaneous | 13 (92.9) | 118 (99.2) | 0.200 |
| Respiratory | 10 (71.4) | 53 (44.5) | 0.088 |
| Cardiovascular | 6 (42.9) | 1 (0.8) | <0.001 |
| Gastrointestinal | 2 (14.3) | 70 (58.8) | 0.003 |
| Neurologic | 2 (14.3) | 1 (0.8) | 0.029 |
| Blood pressure (mmHg) | |||
| Systolic blood pressure | 105 (96–127) | 98 (95–106.5) | 0.155§ |
| Diastolic blood pressure | 72.5 (63.8–80) | 61 (55.5–70) | 0.017∗ |
| Severe symptoms | 2 (14.3) | 2 (1.7) | 0.055 |
| Nonalert consciousness | 1 (7.1) | 0 (0.0) | 0.105 |
| ED treatment | |||
| O2 supply | 2 (14.3) | 4 (3.4) | 0.121 |
| Fluid administration | 13 (92.9) | 41 (34.5) | <0.001 |
| Antihistamine use | 12 (85.7) | 112 (94.1) | 0.241 |
| Steroid use | 9 (64.3) | 36 (30.3) | 0.016 |
| Epinephrine use | 8 (57.1) | 17 (14.3) | 0.001 |
| Bronchodilator use | 6 (42.9) | 18 (15.1) | 0.021 |
Table 5.
Univariate and multivariate logistic regression analysis for factors associated with anaphylaxis registered correctly




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download