Abstract
Helicobacter pylori (H. pylori), a causative agent of chronic gastritis and gastric cancer, has several virulent factors for own survival and progression toward gastric diseases in human stomach. Of those, H. pylori produces mainly urease (10~15% total protein weight) that neutralize the gastric acid for survival. Here, we identified the antigenic epitope of urease and then developed an ELISA using the antigen including the epitope of urease. We identified the antigenic epitope of urease that induces IgA antibodies in human using truncated mutants. Eight kinds of serially-truncated mutant of UreA and UreB were prepared and subjected to immunoblot using pooled sera of patients with gastric disorders. UreBEnd protein containing UreB epitope was produced and investigated its diagnostic value via ELISA in children. As a result, mutants having last 24 amino acid residues of UreB carboxyl terminus deleted did not show IgA-reactive band. The clones that contained the downstream of 448th amino acid in UreB showed IgA-reactive band. The serodiagnostic value of the UreBEnd recombinant protein including identified epitope was confirmed via IgA ELISA and shown to have 97% sensitivity and 100% specificity. These results demonstrated that carboxyl terminal region of UreB carries an antigenic epitope for IgA response in human. It may be useful for detecting H. pyloriinfection with improved test accuracy and minimum use of endoscopy.
REFERENCES
1). Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984; 1:1311–5.

2). Jones KR, Whitmire JM, Merrell DS. A Tale of Two Toxins: Helicobacter pyloriCagA and VacA Modulate Host Pathways that Impact Disease. Front Microbiol. 2010; 1:115.

3). Hatakeyama M. Helicobacter pyloriCagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014; 15:306–16.
4). Salih BA. Helicobacter pyloriinfection in developing countries: the burden for how long? Saudi J Gastroenterol. 2009; 15:201–7.

5). Ohkusa T, Okayasu I, Miwa H, Ohtaka K, Endo S, Sato N. Helicobacter pyloriinfection induces duodenitis and superficial duodenal ulcer in Mongolian gerbils. Gut. 2003; 52:797–803.
6). Jang KM, Choe BH, Choe JY, Hong SJ, Park HJ, Chu MA, et al. Changing Prevalence of Helicobacter pyloriInfections in Korean Children with Recurrent Abdominal Pain. Pediatr Gastroenterol Hepatol Nutr. 2015; 18:10–6.
7). Ben-Hur H, Gurevich P, Elhayany A, Avinoach I, Schneider DF, Zusman I. Transport of maternal immunoglobulins through the human placental barrier in normal pregnancy and during inflammation. Int J Mol Med. 2005; 16:401–7.

8). Veenendaal RA, Götz JM, Schroijen V, Kurban F, Bernards AT, Veselic M, et al. Diagnosis of Helicobacter pyloriinfection by specific gastric mucosal IgA and IgG pylori antibodies. J Clin Pathol. 1995; 48:990–3.
9). IARC. Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994; 61:1–241.
10). Roesler BM, Rabelo-Goncalves EM, Zeitune JM. Virulence Factors of Helicobacter pylori: A Review. Clin Med Insights Gastroenterol. 2014; 7:9–17.

11). Wu C, Zou QM, Guo H, Yuan XP, Zhang WJ, Lu DS, et al. Expression, purification and immuno-characteristics of recombination UreB protein of H. pylori. World J Gastroenterol. 2001; 7:389–93.
12). Hu LT, Mobley HL. Purification and N-terminal analysis of urease from Helicobacter pylori. Infect Immun. 1990; 58:992–8.

13). Seo JH, Park JS, Yeom JS, Lim JY, Park CH, Woo HO, et al. Correlation between positive rate and number of biopsy samples on urease test in childhood Helicobacter pyloriinfection. J Korean Med Sci. 2014; 29:106–9.
14). Baik SC, Youn HS, Chung MH, Lee WK, Cho MJ, Ko GH, et al. Increased oxidative DNA damage in Helicobacter pylori-infected human gastric mucosa. Cancer Res. 1996; 56:1279–82.
15). Ha NC, Oh ST, Sung JY, Cha KA, Lee MH, Oh BH. Supramolecular assembly and acid resistance of Helicobacter pylori urease. Nat Struct Biol. 2001; 8:505–9.
16). Minkara MS, Ucisik MN, Weaver MN, Merz KM Jr. Molecular Dynamics Study of Helicobacter pyloriUrease. J Chem Theory Comput. 2014; 10:1852–62.
17). Ghalehnoei H, Ahmadzadeh A, Farzi N, Alebouyeh M, Aghdaei HA, Azimzadeh P, et al. Relationship between ureB Sequence Diversity, Urease Activity and Genotypic Variations of Different Helicobacter pyloriStrains in Patients with Gastric Disorders. Pol J Microbiol. 2016; 65:153–9.
Figure 2.
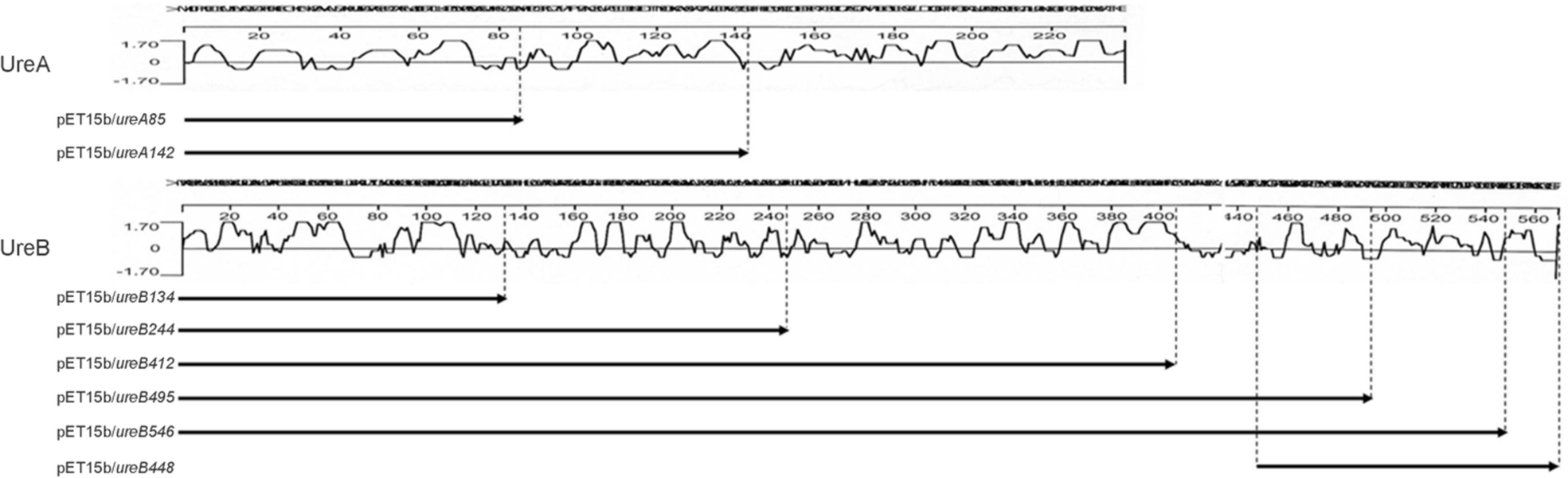
Reactivities of human antisera to truncated mutant proteins of UreA (A) and UreB (B). The truncated mutant proteins of UreA and UreB were expressed from pET15b clones. Reactivity of the proteins were analyzed by patient's sera. Lane M, molecular weight markers; 1, UreA; 2, UreA142; 3, UreA85; 4, UreB; 5, UreB546; 6, UreB495; 7, UreB412; 8, UreB244; 9, UreB134; 10, UreB448.
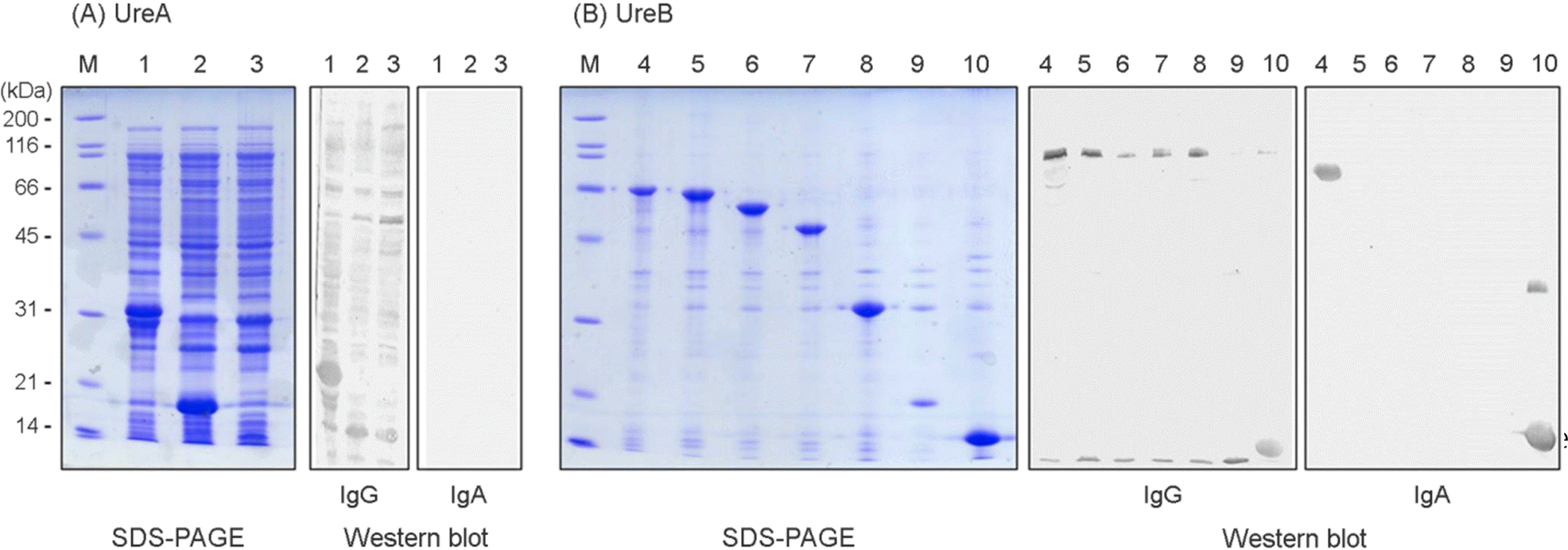
Figure 3.
Production of recombinant UreBEnd protein. The recombinant UreBEnd protein was purified from pEGexs/UreBEnd clone by Glutathione column. Reactivity of the UreBEnd protein was analyzed with patients' pooled sera. Lane M, molecular weight markers; 1, Non-induction; 2, the whole cell lysate after IPTG induction; 3, the supernatant of whole cell lysate after IPTG induction; 4, the pellets of whole cell lysate after IPTG induction; 5, elution; 6, thrombin treated elution; 7, vector control;8~11, elution 1~4.
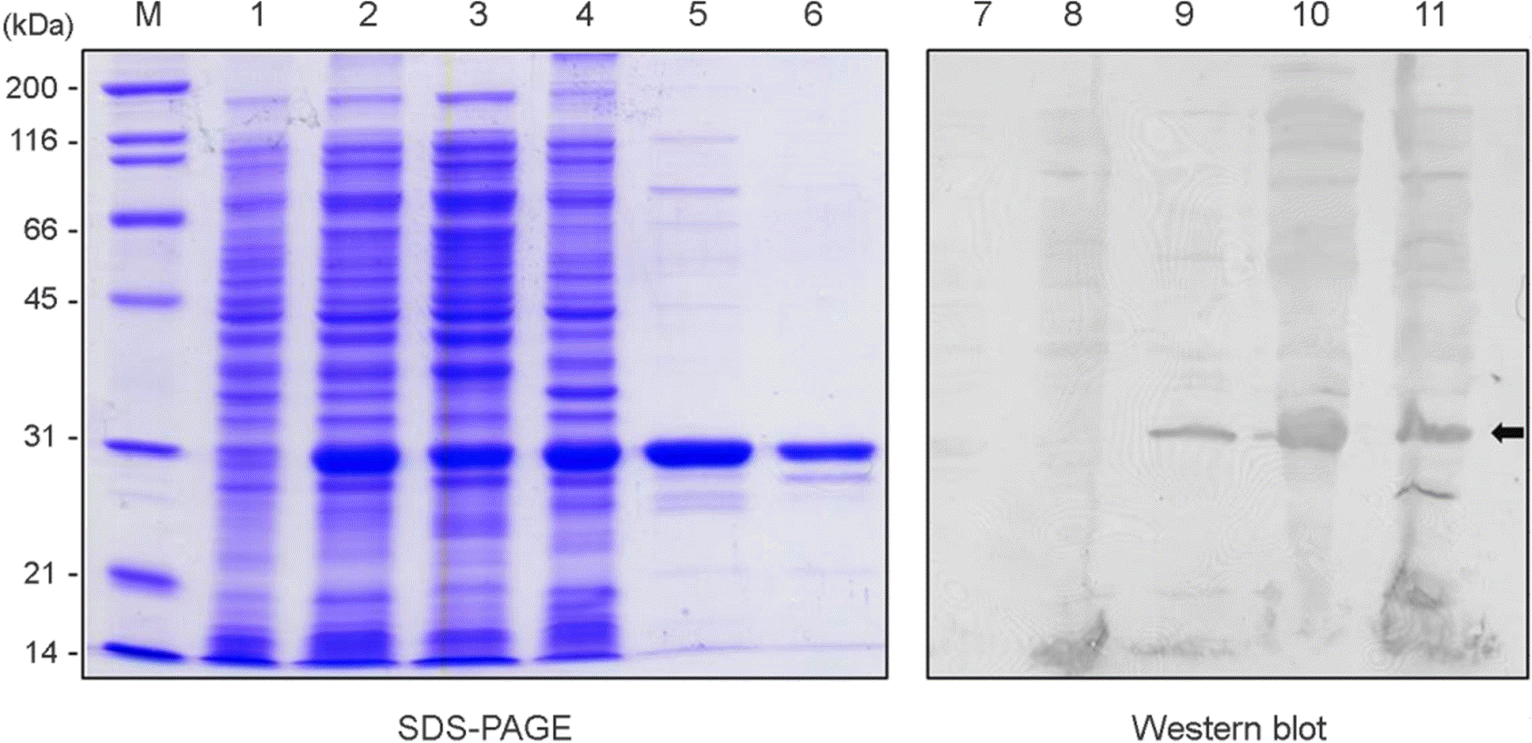
Figure 4.
Location and sequence of antigenic determinant of UreB. Colored underlined sequence denotes the epitope sequence. Underlined numbers indicate 3′-termini's amino acid site of truncated mutant proteins.
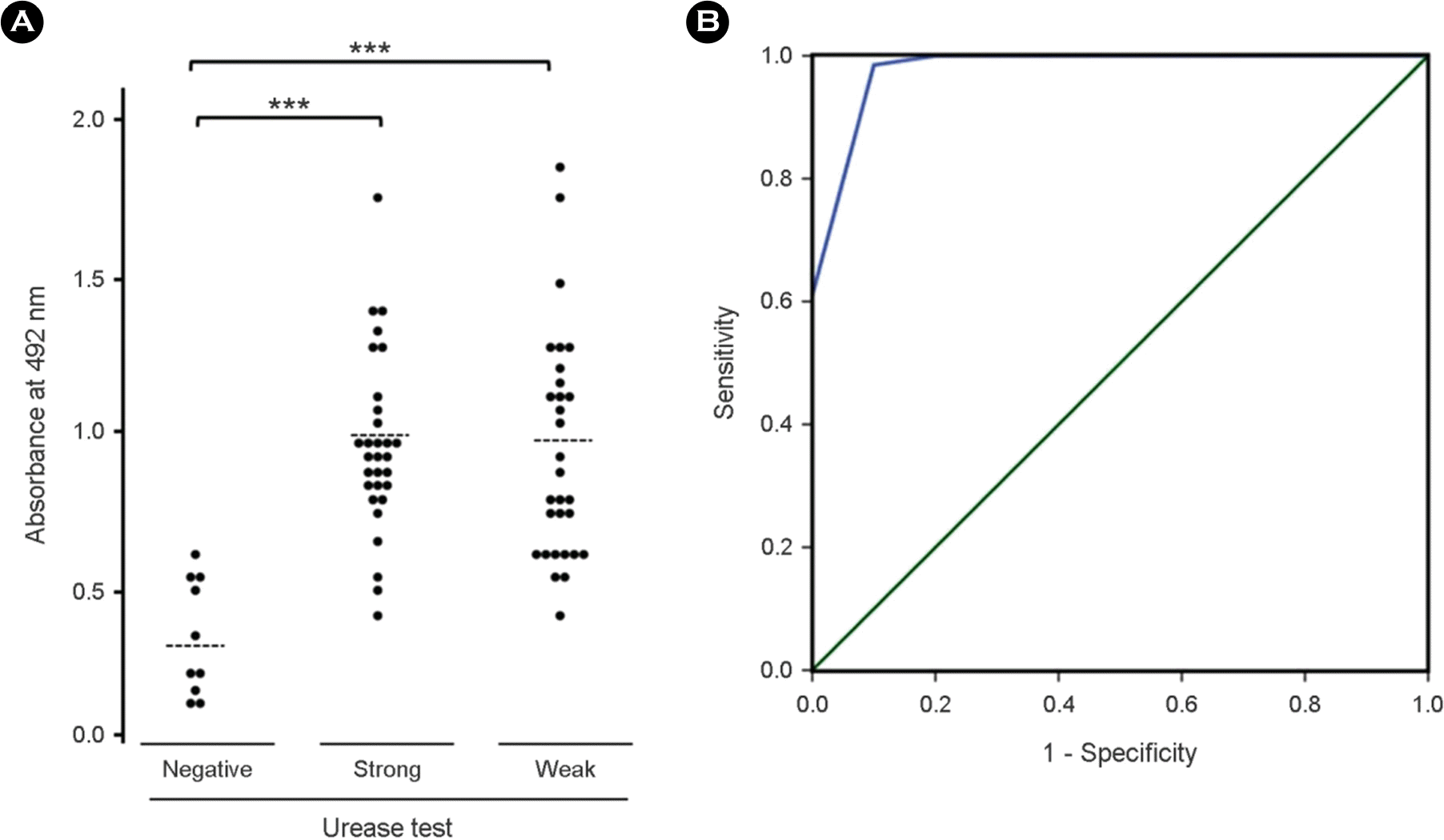
Figure 5.
IgA distribution of sera from the patients with/without gastric symptoms measured by ELISA using purified recombinant UreBEnd. (A) Dashed line indicates the average of absorbance value: negative, 0.35; strong, 0.96; weak reactivity to CLO test, 0.95. (B) Receiver operating characteristic (ROC) curves of UreBEnd ELISA for the diagnosis of H. pyloriinfection. The area under the ROC of UreBEnd ELISA was 0.997 (95% confidence interval; p< 0.001).
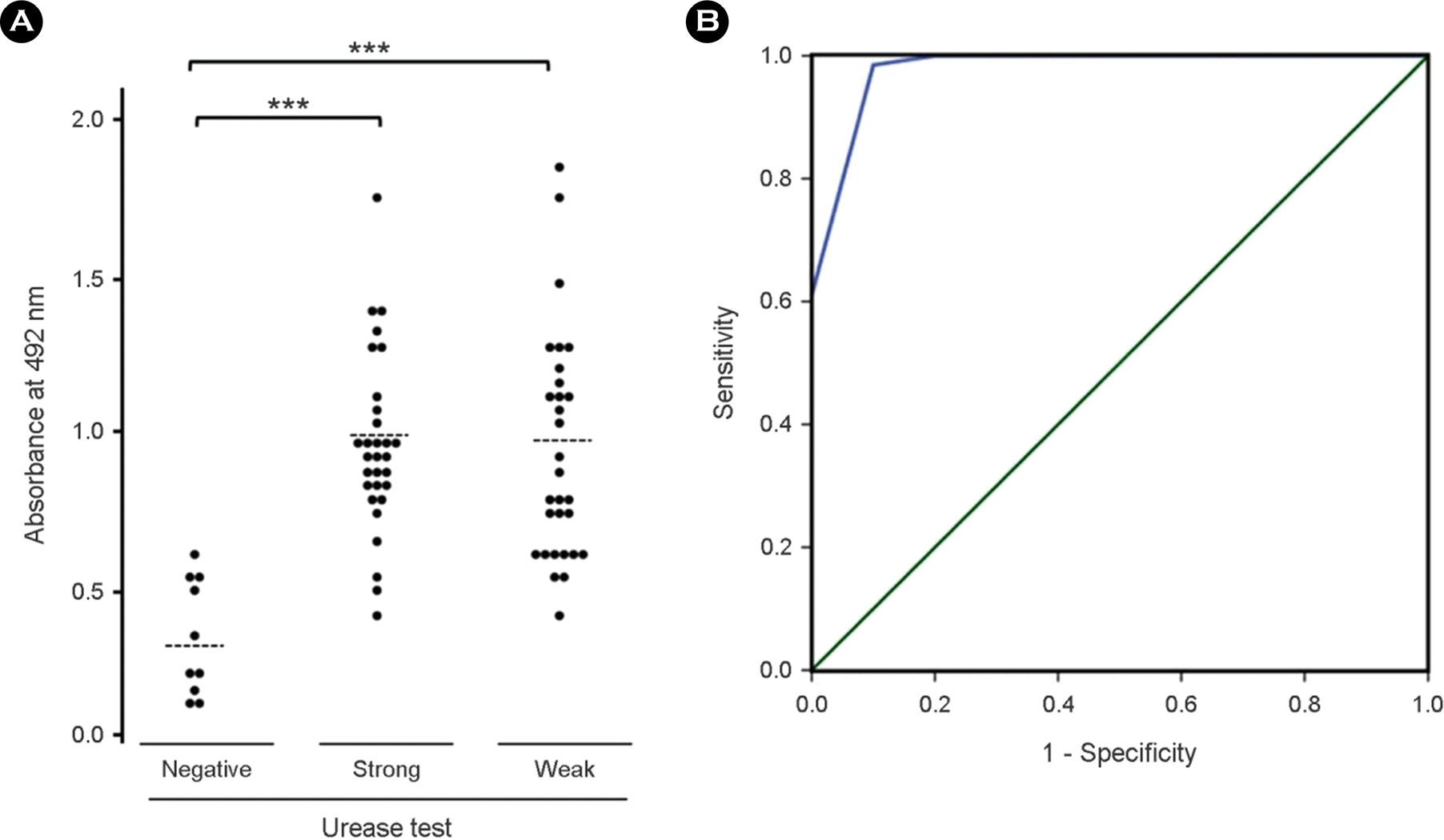
Table 1.
Oligonucleotide primers for PCR amplification of ureA and ureBfragments




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download