Abstract
Among a myriad of pathogen-associated molecular pattern-sensing receptors, toll-like receptors (TLRs) are the principal core sensors of the host. Despite intensive studies for the expression of TLRs and their roles in the central nervous system, controversies remain regarding the expression and the function of TLR4 in human astrocytes. In order to clarify this issue, we attempted to verify functional expression of TLR4 in human astrocytes. Using Reverse transcription-polymerase chain reaction (RT-PCR), we confirmed that the human astrocytes express the TLR4 constitutively. To determine the function of TLR4, astrocytes were treated with TLR4 ligand or lipopolysaccharide (LPS), and then inflammatory cytokines expressions were checked using RT-PCR and enzyme-linked immunosorbent assay. Nuclear factor (NF)-κB activation was checked using electrophoretic mobility shift assay. Treatment of astrocytes with LPS increased tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8 expression and induced NF-κB activation. Neutralizing anti-TLR4 antibody blocked the effect of LPS on cytokine production and NF-κB activation in astrocytes. The effect of LPS on cytokine production and NF-κB activation was shown in the presence of serum but not in the absence of serum. Therefore, we investigated the sensing mechanism of LPS in human astrocytes. Human astrocytes were treated with LPS following neutralizing anti-CD14 antibody treatment in the presence of serum. Neutralizing anti-CD14 antibody treatment abolished the effect of LPS on cytokine expression and NF-κB activation. Additionally, supplement of recombinant CD14 in serum-free media induced LPS effect on cytokine production and NF-κB activation. In these results, we showed that human astrocytes constitutively express functional TLR4 and require soluble CD14 to recognize LPS.
REFERENCES
1). Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Disproportionate recruitment of CD8+ T cells into the central nervous system by professional antigen-presenting cells. Am J Pathol. 1999; 154:481–94.

2). Hua LL, Lee SC. Distinct patterns of stimulus-inducible chemokine mRNA accumulation in human fetal astrocytes and microglia. Glia. 2000; 30:74–81.

3). Lee SC, Dickson DW, Liu W, Brosnan CF. Induction of nitric oxide synthase activity in human astrocytes by interleukin-1 beta and interferon-gamma. J Neuroimmunol. 1993; 46:19–24.
4). Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003; 43:281–91.

5). Kielian T. Overview of toll-like receptors in the CNS. Curr Top Microbiol Immunol. 2009; 336:1–14.

6). Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005; 49:360–74.

7). Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005; 159:12–9.

8). Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, et al. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002; 22:2478–86.

9). Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001; 2:675–80.

11). Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005; 17:1–14.
12). Bohr V, Paulson OB, Rasmussen N. Pneumococcal meningitis. Late neurologic sequelae and features of prognostic impact. Arch Neurol. 1984; 41:1045–9.
14). Durand ML, Calderwood SB, Weber DJ, Miller SI, Southwick FS, Caviness VS Jr, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993; 328:21–8.
15). Quagliarello V, Scheld WM. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992; 327:864–72.

16). Holm TH, Draeby D, Owens T. Microglia are required for astroglial Toll-like receptor 4 response and for optimal TLR2 and TLR3 response. Glia. 2012; 60:630–8.

17). Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003; 100:8514–9.

18). Hao HN, Zhao J, Lotoczky G, Grever WE, Lyman WD. Induction of human beta-defensin-2 expression in human astrocytes by lipopolysaccharide and cytokines. J Neurochem. 2001; 77:1027–35.
19). Lafortune L, Nalbantoglu J, Antel JP. Expression of tumor necrosis factor alpha (TNF alpha) and interleukin 6 (IL-6) mRNA in adult human astrocytes: comparison with adult microglia and fetal astrocytes. J Neuropathol Exp Neurol. 1996; 55:515–21.
20). Lee SS, Seo HS, Choi SJ, Park HS, Lee JY, Lee KH, et al. Characterization of the two genes differentially expressed during development in human fetal astrocytes. Yonsei Med J. 2003; 44:1059–68.

21). Choi C, Park JY, Lee J, Lim JH, Shin EC, Ahn YS, et al. Fas ligand and Fas are expressed constitutively in human astrocytes and the expression increases with IL-1, IL-6, TNF-alpha, or IFN-gamma. J Immunol. 1999; 162:1889–95.
22). Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983; 11:1475–89.

23). Benveniste EN, Benos DJ. TNF-alphaand IFN-gamma-mediated signal transduction pathways: effects on glial cell gene expression and function. FASEB J. 1995; 9:1577–84.
24). Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992; 263:C1–16.

25). Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002; 61:1013–21.

26). Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005; 175:4320–30.

27). de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, et al. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996; 64:37–43.

28). Hauss-Wegrzyniak B, Lukovic L, Bigaud M, Stoeckel ME. Brain inflammatory response induced by intracerebroventricular infusion of lipopolysaccharide: an immunohistochemical study. Brain Res. 1998; 794:211–24.

29). Mayhan WG. Cellular mechanisms by which tumor necrosis factor-alpha produces disruption of the blood-brain barrier. Brain Res. 2002; 927:144–52.
30). Schumann RR, Pfeil D, Freyer D, Buerger W, Lamping N, Kirschning CJ, et al. Lipopolysaccharide and pneumococcal cell wall components activate the mitogen activated protein kinases (MAPK) erk-1, erk-2, and p38 in astrocytes. Glia. 1998; 22:295–305.

31). Cauwels A, Frei K, Sansano S, Fearns C, Ulevitch R, Zimmerli W, et al. The origin and function of soluble CD14 in experimental bacterial meningitis. J Immunol. 1999; 162:4762–72.
32). Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006; 53:688–95.

Figure 1.
The expression of TLRs in human astrocytes (HA). Total RNA was isolated and RT-PCR for TLR1-9 was performed in the astrocytes and THP-1 cells (A). After LPS treatment for 7 h at variable dosages in HA, total RNA was isolated and RT-PCR for TLR2 was performed (B).
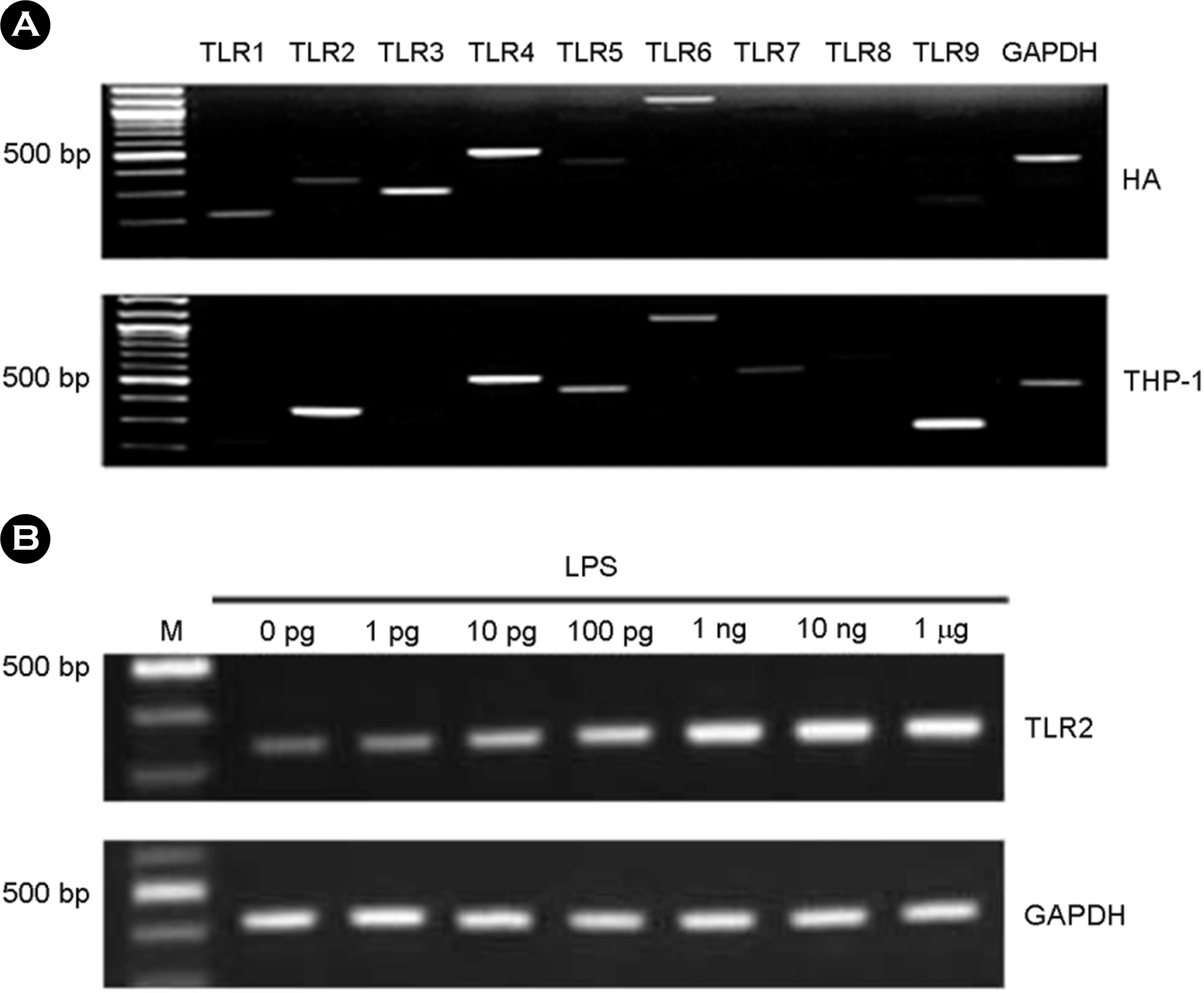
Figure 2.
The effect of LPS on cytokine production in astrocytes. The astrocytes were cultured in DMEM containing 10% serum. After LPS treatment for 6 h at variable dosages, total RNA was isolated and RT-PCR for TNF-α, IL-6 and GAPDH was per-formed (A). The astrocytes were incubated with LPS or 1 ng/ml of IL-1β for 24 h, and culture supernatants were then harvested. The supernatants were assayed for immunoreactive IL-6 (B) and IL-8 (C) using ELISA. ∗, p < 0.05
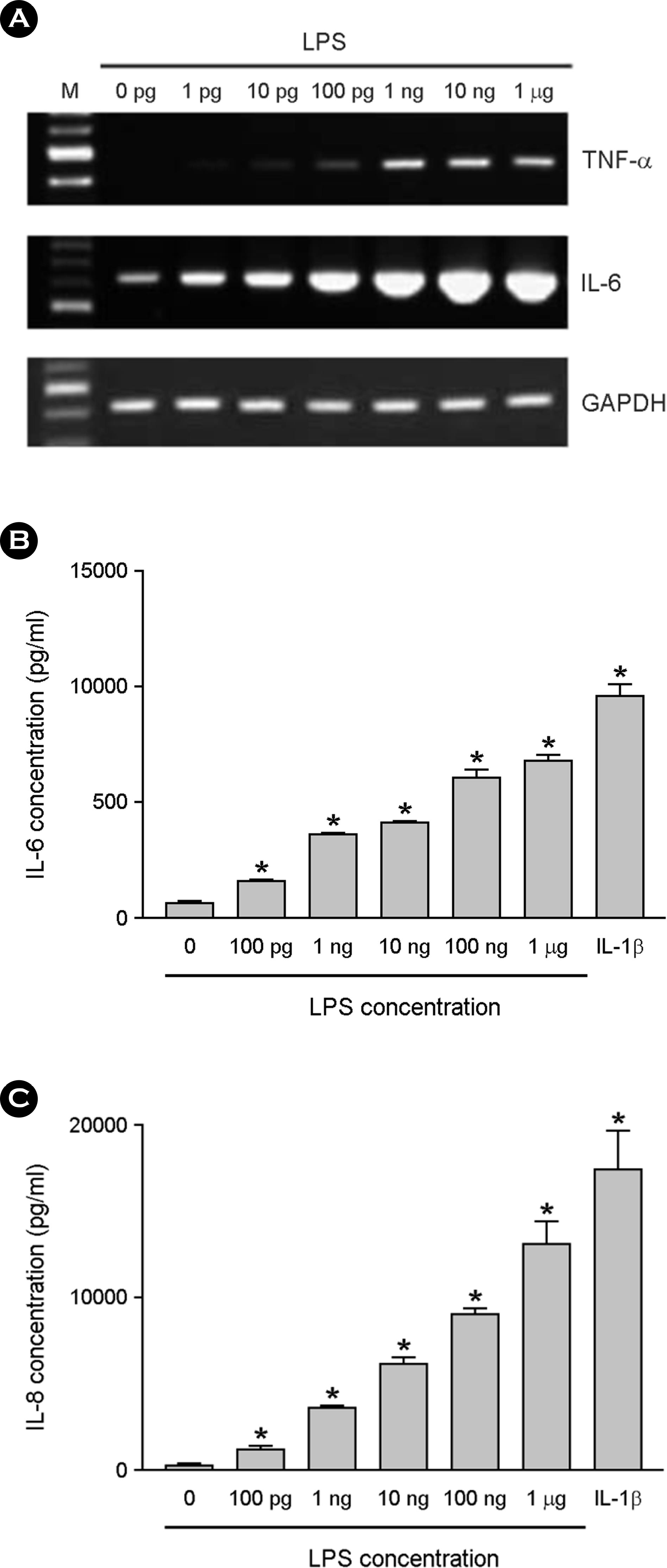
Figure 3.
The effect of LPS on cytokine expression in the absence of serum. The astrocytes were treated with LPS for 6 h in serum free DMEM. Total RNA was isolated and RT-PCR for TNF-α was performed (A). The cultures were incubated with LPS or 1 ng/ ml of IL-1β for 24 h in serum free media, and culture supernatants were then harvested. The supernatants were assayed for immunoreactive IL-6 (B) and IL-8 (C) using ELISA. ∗, p < 0.05
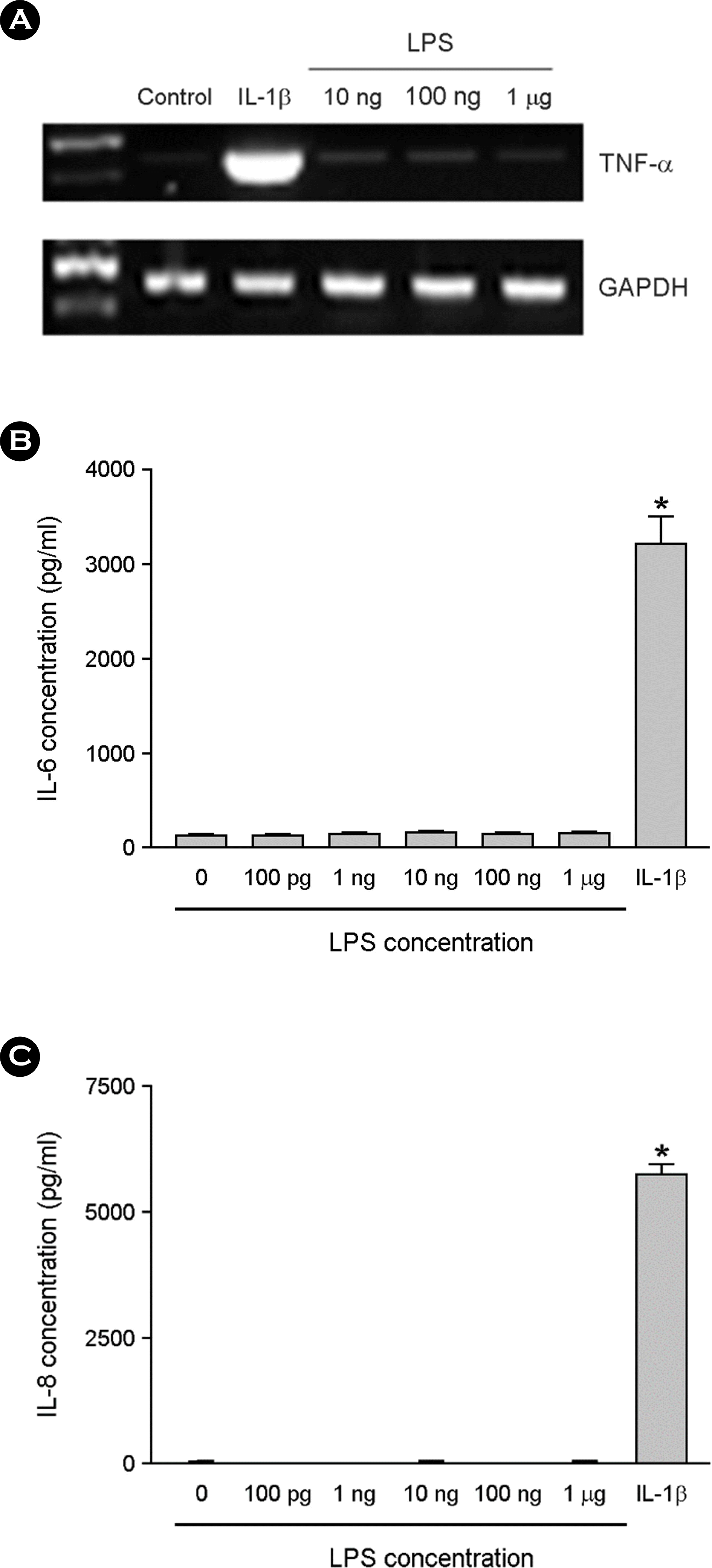
Figure 4.
The serum effect on NF-κB activation in LPS-treated astrocytes. Cells were incubated with LPS (100 ng/ml) in the presence or absence of serum. After incubation for the indicated amount of time, nuclear proteins were extracted and EMSA for NF-κB was performed.
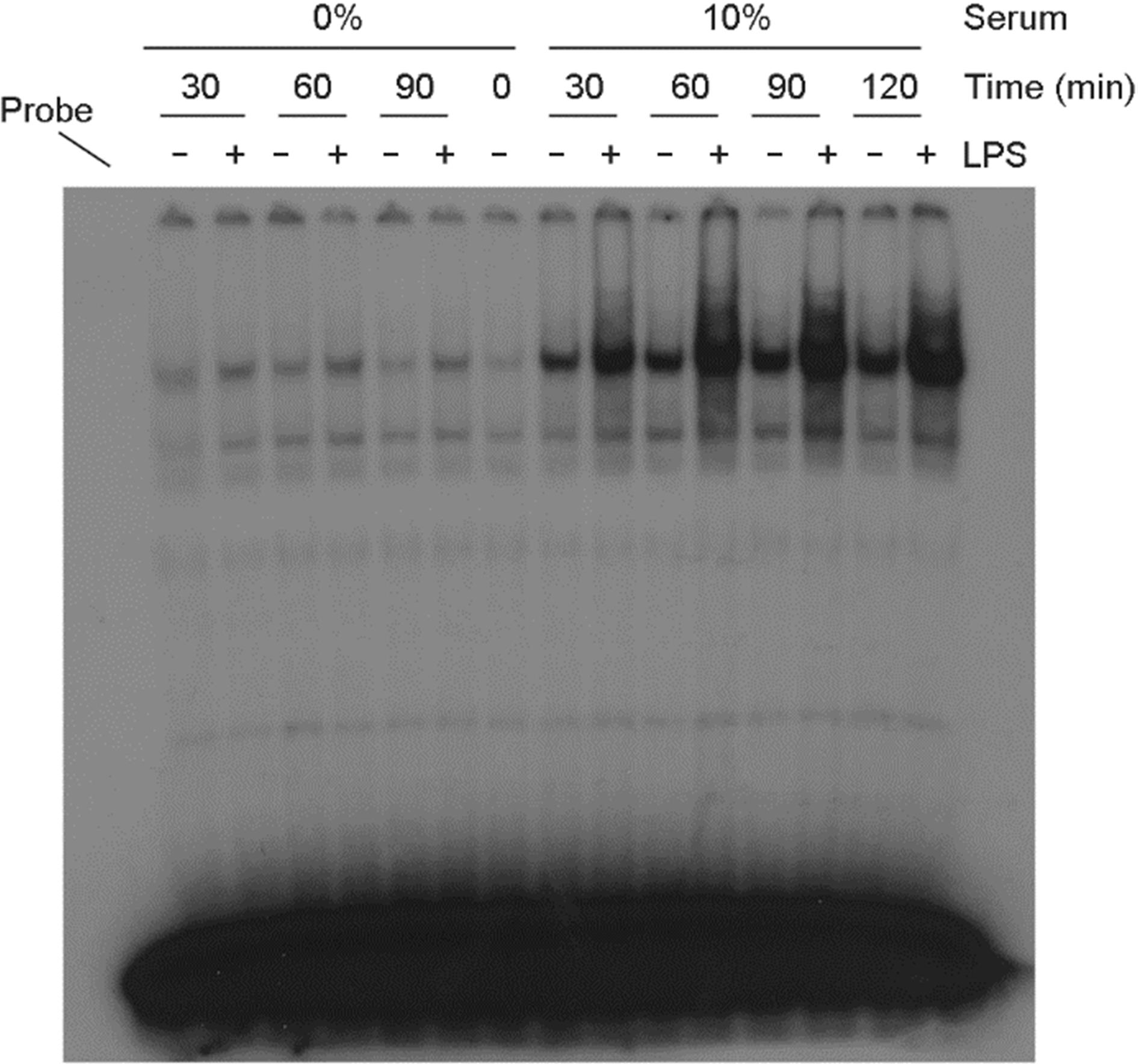
Figure 5.
The effect of sCD14 supplementation on LPS-induced cytokine production in astrocytes. The cells were incubated with indicated doses of sCD14 and 10 ng/ml of LPS for 24 h in serum free media. The culture supernatants were harvested and assessed for IL-6 production using ELISA (A). The cells were treated with different concentrations of LPS or 10 ng/ml of IL-1β in the presence or absence of 0.1 μg/ml of sCD14. After incubation for 24 h, the culture supernatants were harvested and assessed for IL-6 production (B) or IL-8 production (C) using ELISA. ∗, p < 0.05
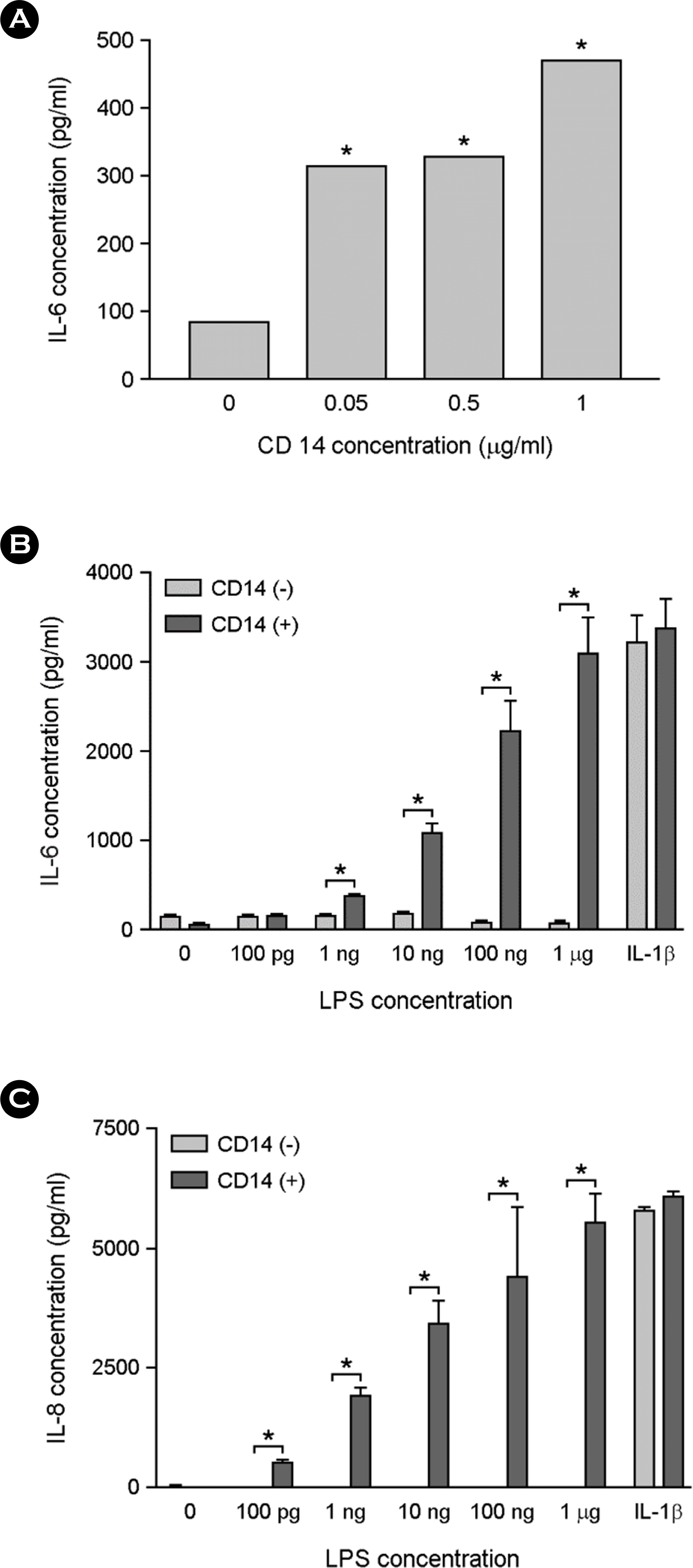
Figure 6.
The NF-κB activation induced by LPS treatment is dependent on sCD14. The cells were incubated with 10 ng/ml of LPS or 1 μg/ml of sCD14 or both for 30 min in serum free media. After nuclear extracts were harvested, EMSA for NF-κB was performed. (A). The cells were incubated with or without 5 μg/ml of neutralizing anti-CD14 antibodies in serum containing media for 1 h. Ten ng/ml of LPS was added. After incubation for 30 min, nuclear extracts were harvested, and EMSA for NF-κB was performed. (B).
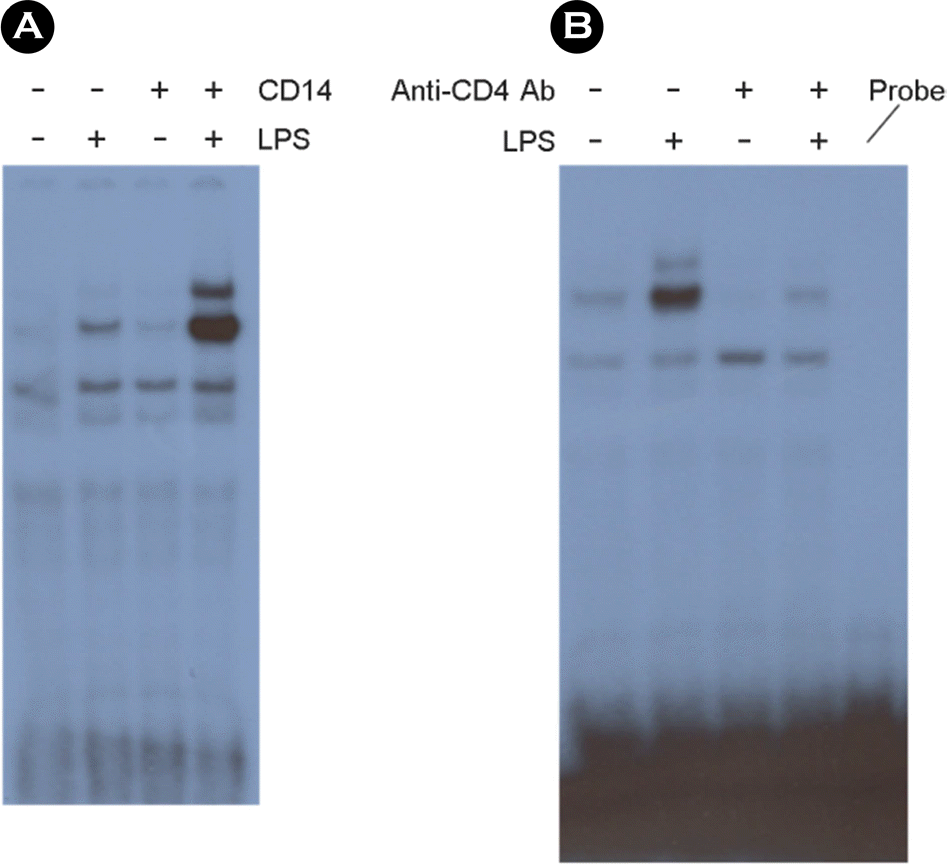
Figure 7.
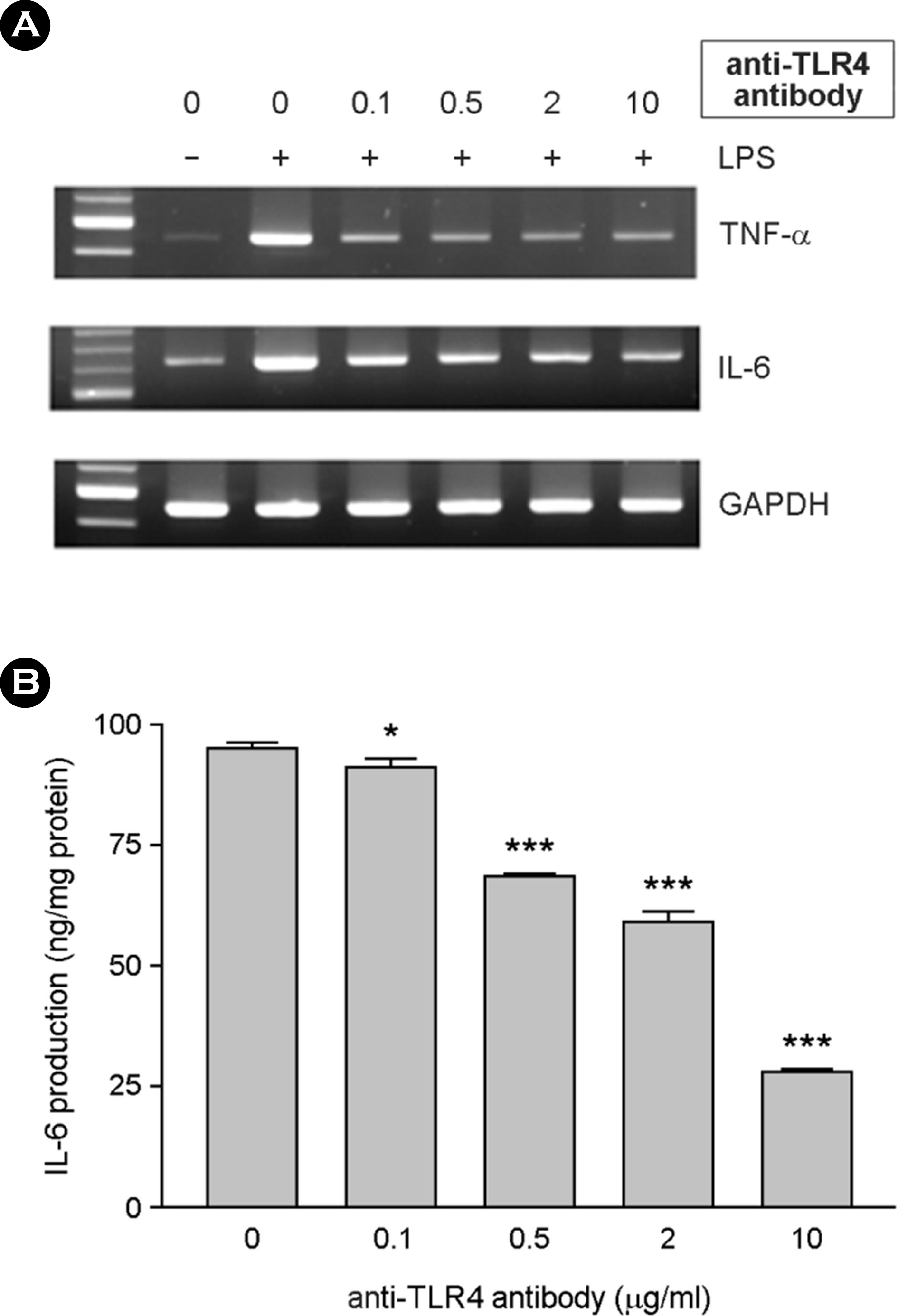
The effect of LPS-inducing cytokine expression was inhibited by anti-TLR4 antibody. Serum-free DMEM containing 0.1 μg/ml of sCD14 was used for the experiments. Cells were incubated with 0.1, 0.5, 2 and 10 μg/ml of anti-TLR4 antibody for 1 hr. Ten ng/ml of LPS was added. After incubation for 6 h, total RNAs were isolated and used for RT-PCR for TNF-α and IL-6 (A). After incubation for 24 h, culture supernatants were harvested and IL-6 production was assessed using ELISA (B). ∗, p < 0.05, ∗∗∗, p < 0.001




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download