Abstract
Mycobacterium tuberculosis likely reside within a granuloma as a dormant state. An area of necrosis forms at the center of lung granulomas. Within this area, the bacteria are deprived of nutrients and exposed to harsh conditions, including low pH and anoxia. The response of M. tuberculosis to low pH and low oxygen conditions was investigated in both cellular and extracellular proteins by two-dimensional polyacrylamide gel electrophoresis analysis and MALDI-TOF. Several proteins intensively expressed under low pH and/or hypoxic conditions were found. In the culture filtrate, PhoS1 (Rv0934) and ScoB (Rv2503c) were found in significant amounts under both the low oxygen and acidic stress conditions. These results indeed extend our understanding of acidic response as well as hypoxic in M. tuberculosis and provide an important insight into physiology of the latent bacilli.
References
1). 박정규, 장희철, 임재현, 송창화, 김운옥, 조은경, 김화 중. 결핵균 배양액에서 38 kDa 당지질 단백항원의 정 제와 분석. J Bacteriol Virol. 31:249–257. 2001.
2). Barker LP, George KM, Falkow S, Small PLC. Differential trafficking of live and dead Mycobacterium marinum organisms in macrophages. Infect Immun. 65:1497–1504. 1997.
3). Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 43:717–731. 2002.
4). Bentrup KH, Russell DG. Mycobacterial persistence: adaptation to a changing environment. Trends Microbiol. 9:597–605. 2001.

5). Boon C, Dick T. Mycobacterium bovis BCG response regulator essential for hypoxic dormancy. J Bacteriol. 184:6760–6767. 2002.
6). Braibant M, Lefevre P, Wit L, Peirs P, Ooms J, Huygen K, Andersen AB, Content J. A Mycobacterium tuberculosis gene cluster encoding proteins of a phosphate transporter homologous to the Escherichia coli Pst system. Gene. 176:171–176. 1996.
7). Buchmeier N, Blanc-Potard A, Ehrt S, Piddington D, Riley L, Groisman EA. A parallel intraphagosomal survival strategy shared by Mycobacterium tuberculosis and Salmonella enterica. Mol Microbiol. 35:1375–1382. 2000.
8). Chiang IH, Suo J, Bai KJ, Lin TP, Luh KT, Yu CJ, Yang PC. Serodiagnosis of Tuberculosis. Am J Respir Crit Care Med. 156:906–911. 1997.

9). Corthesy-Theulaz IE, Bergonzelli GE, Henry H, Bachmann D, Schorderet DF, Blum AL, Ornston LN. Cloning and Characterization of Helicobacter pylori succinyl CoA: aceto-acetate CoA-transferase, a Novel prokaryotic member of the CoA-transferase Familiy. J Biol Chem. 272:25659–25667. 1997.
10). Dick T. Dormant tubercle bacilli: the key to more effective TB chemotherapy? J Antimicrob Chemother. 47:113–124. 2001.

11). Dick T, Lee BH, Murugasu-Oei B. Oxygen deletion induced dormancy in Mycobacterium smegmatis. FEMS Microbiol Lett. 163:159–164. 1998.
12). Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. J Am Med Assoc. 282:677–686. 1999.
13). Fenton MJ, Vermeulen MW. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect Immun. 64:683–690. 1996.

14). Fisher MA, Plikaytis BB, Shinnick TM. Microarray analysis of the Mycobacterium tuberculosis transcriptional response to the acidic conditions found in phagosomes. J Bacteriol. 184:4025–4032. 2002.
15). Gomes MS, Paul S, Moreira AL, Appelberg R, Rabinovitch M, Kaplan G. Survival of Mycobacterium avium and Mycobacterium tuberculosis in acidified vacuoles of murine macrophages. Infect Immun. 67:3199–3206. 1999.
16). Hu YM, Butcher PD, Sole K, Mitchison DA, Coates ARM. Protein synthesis is shutdown in dormant Mycobacterium tuberculosis and is reversed by oxygen or heat shock. FEMS Microbiol Lett. 158:139–145. 1998.
17). Actor Jeffrey K., Olsen Margaret, Chinnaswamy Jagannath, Hunter Robert L.Relationship of survival, organism containment, and granuloma formation in acute murine tuberculosis. J Interferon Cytokine Res. 19:1183–1193. 1998.

18). Lefevre P, Braibant M, Wit LD, Kalai M, Roeper D, Grotzinger J, Delville JP, Peirs P, Ooms J, Huygen K, Content J. Three different putative phosphate transport receptors are encoded by the Mycobacterium tuberculosis genome an are present at the surface of Mycobacterium bovis BCG. J Bacteriol. 179:2900–2906. 1997.
19). Lim A, Eleuterio M, Hutter B, Murugasu-Oei B, Dick T. Oxygen depletion-induced dormancy in Mycobacterium bovis BCG. J Bacteriol. 181:2252–2256. 1999.
20). Manabe YC, Bishai WR. Latent Mycobacterium tuberculosis-persistent, patience, and winning by waiting. Nat Med. 6:1327–1329. 2000.
21). Mariani F, Cappelli G, Riccardi G, Colizzi V. Mycobacterium tuberculosis H37Rv comparative gene-expression analysis in synthetic medium and human macrophage. Gene. 253:281–291. 2000.
22). Monahan IE, Betts J, Banerjee DK, Butcher PD. Differential expression of mycobacterial proteins following phagocytosis by macrophages. Microbiology. 147:459–471. 2001.

23). Oh YK, Straubinger RM. Intracellular fate of Mycobacterium avium: Use of dual-label spectrofluorometry to investigate the influence of bacterial viability and opsonization on phagosomal pH and phagosome-lysosome interaction. Infect Immun. 64:319–325. 1996.
24). Parrish NM, Dick J, Bishai WR. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 6:107–112. 1998.
25). Piddington DL, Kashkouli A, Buchmeier NA. Growth of Mycobacterium tuberculosis in a defined medium is very restricted by acid pH and Mg2+ levels. Infect Immun. 68:4518–4522. 2000.
26). Quinn FD, Birkness KA, King PJ. Alpha-crystallin as a potential marker of Mycobacterium tuberculosis latency. ASM News. 68:612–617. 2002.
27). Rosenkrands I, Slayden RA, Crawford J, Aagaard C, Barry CE 3rd, Andersen P. Hypoxic response of Mycobacterium tuberculosis studied by metabolic labeling and proteome analysis of cellular and extracellular proteins. J Bacteriol. 184:3485–3491. 2002.
28). Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, Monahan IM, Dolganov G, Efron B, Butcher PD, Nathan C, Schoolnik GK. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J Exp Med. 198:693–704. 2003.
29). Selwyn PA, Hartel D, Lewis VA, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 320:545–550. 1989.
30). Solca NM, Bernasconi MV, Valsangiacomo C, Van Doorn LJ, Piffaretti JC. Population genetics of Helicobacter pylori in the southern part of Switzerland analysed by sequencing of four housekeeping genes (atpD, glnA, scoB and recA), and by vacA cag, A, iceA and IS605 genotyping. Microbiology. 147:1693–1707. 2001.
31). Starck J, Kallenius G, Marklund BI, Andersson DI, Akerlund T. Comparative proteome analysis of Mycobacterium tuberculosis grown under aerobic and anaerobic conditions. Microbiology. 150:3821–3829. 2004.
32). Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, Allen RD, Gluck SL, Heuser J, Russell DG. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 263:678–681. 1994.
33). Thangaraj HS, Bull TJ, Smet KAL, Hill MK, Rouse DA, Moreno C, Ivanyi J. Duplication of genes encoding the immunodominant 38 kDa antigen in Mycobacterium intra-cellulare. FEMS Microbiol Lett. 144:235–240. 1996.
34). Torres A, Juarez MD, Cervantes R, Espitia C. Molecular analysis of Mycobacterium tuberculosis phosphate specific transport Characterization of recombinant 38 kDa. Microb Pathog. 30:289–297. 2001.
35). Tufariello JM, Chan J, Flynn JL. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis. 3:578–590. 2003.

36). Wayne LG, Hayes LG. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun. 64:2062–2069. 1996.
37). Werngren J, Hoffner SE. Drug-susceptible Mycobacterium tuberculosis Beijing genotype does not develop mutation-conferred resistance to rifampin at an elevated rate. J Clin Microbiol. 41:1520–1524. 2003.
38). Wilkson RJ, Haslov K, Rappuoli R, Giovannoni F, Narayanan PR, Desai CR, Vordermeier HM, Paulsen J, Pasvol G, Ivanyi J, Singh M. Evaluation of the recombinant 38-kilodalton antigen of Mycobacterium tuberculosis as a potential immunodiagnostic reagent. J Clin Microbiol. 35:553–557. 1997.
39). Xu S, Cooper A, Sturgill-Koszycki S, Heyningen T, Chatterjee D, Orme I, Allen P, Russell DG. Intracelllular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 153:2568–2578. 1994.
40). Yuan Y, Crane DD, Barry CE 3rd. Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial α-crystallin homolog. J Bacteriol. 178:4484–4492. 1996.
Figure 1.
Two-dimensional electrophoretic analysis of the culture filtrate (CF) of M. tuberculosis H37Rv grown under 20% (A), 13% (B), and 0% (C) oxygen conditions. The CF proteins were separated by isoelectric focusing (pH 4–7) in the first dimension and by SDS-PAGE (15% polyacrylamide gel) in the second dimension. The gels were stained with 0.25% Coomassie brilliant blue R250.
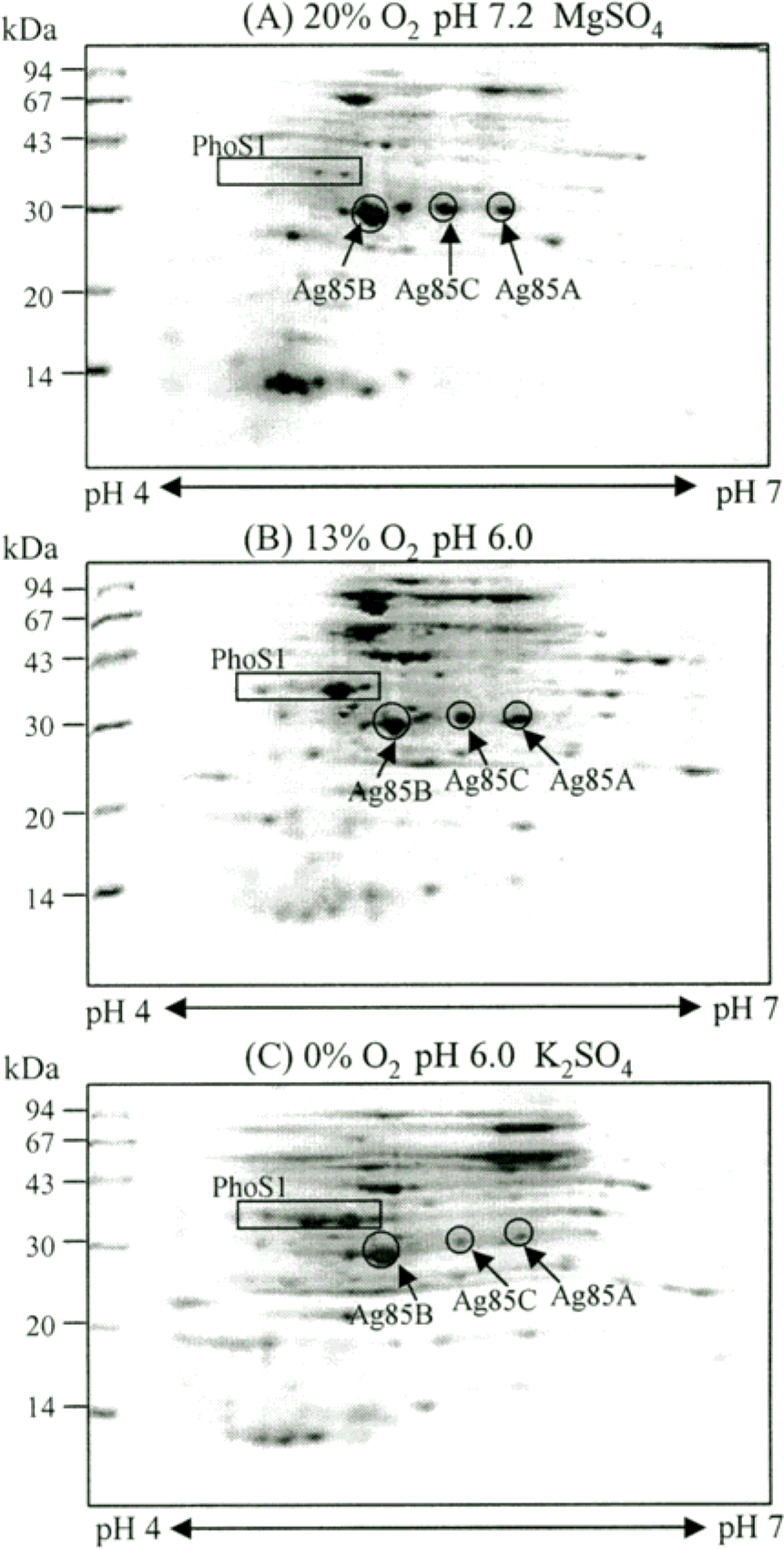
Figure 2.
2-DE analysis of the lysate of M. tuberculosis H37Rv grown under 20% (A), 13% (B), and 0% (C) oxygen conditions. The lysate proteins were separated by isoelectric focusing (pH 4–7) in the first dimension and by 15% SDS-PAGE in the second dimension. The gels were stained with Coomassie brilliant blue R250.
Figure 3.
2-DE analysis of the CF of M. tuberculosis H37Rv grown at different oxygen and pH conditions. The CF proteins were separated by isoelectric focusing (pH 4.7–5.9) in the first dimension and by SDS-PAGE (12% polyacryl-amide gel) in the second dimension. The second dimensional gels were stained with Coomassie brilliant blue R250. The bacteria were cultured with an oxygen supply of 20% (A), 13% (B), and 0% (C), respectively. PhoS1 and ScoB are highly induced under low oxygen and low pH conditions than other dormancy induced protein spots. The numbers on the left indicate molecular mass markers and pI values at the bottom of the panel.
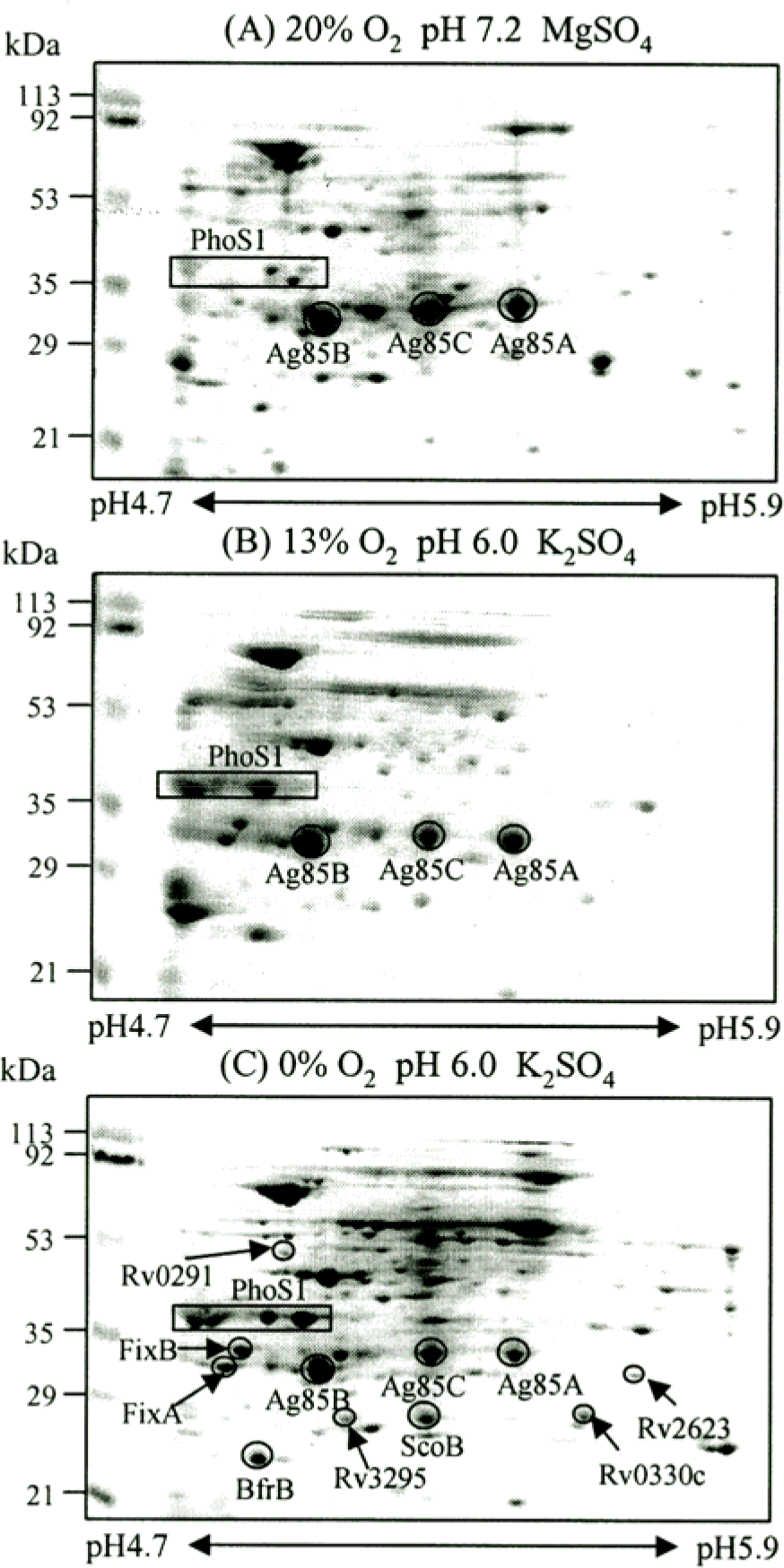
Figure 4.
Analysis of 38 kDa antigen with Coomassie blue staining and Western blot. A. Purified 38 kDa antigen separated by IEF on pH 4.7–5.9 IPG strip in the first dimension and 12% SDS-PAGE in the second dimension. The gel was stained by Coomassie blue. B. The CF obtained from M. tuberculosis grown in pH 6.0, 0% oxygen tension (b) and pH 7.2, 20% oxygen tension 20 (a) were separated by isoelectric focusing (pH 4.7 to 5.9) and SDS-PAGE (12% polyacrylamide gel), and then analyzed by immunoblotting with rabbit anti-38 kDa polyclonal antibody.
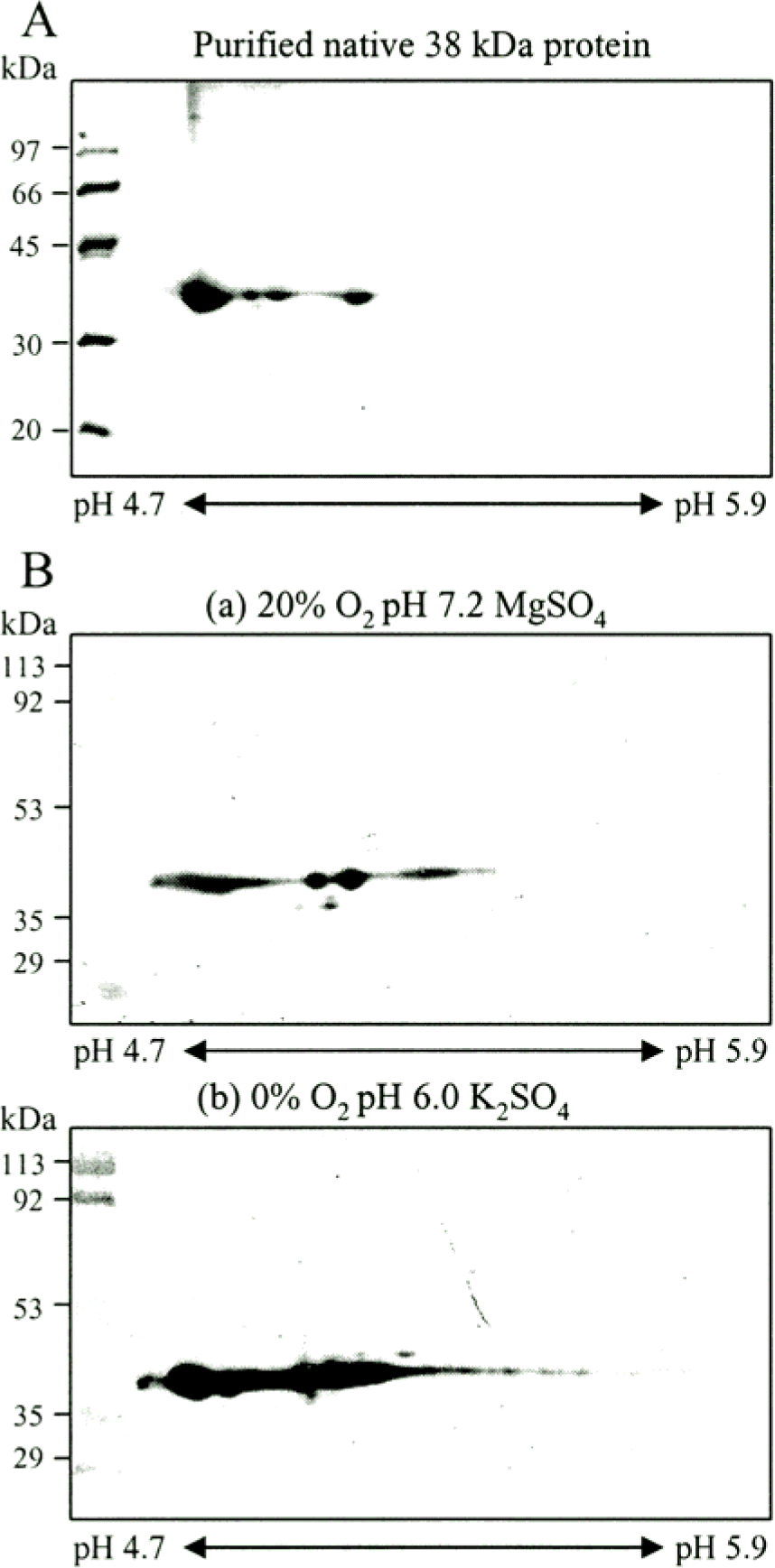
Figure 5.
SDS-PAGE analysis of CF of M. tuberculosis H37Rv grown at 8 different conditions. The CF proteins were separated first dimension by 12% SDS-PAGE. Empty box indicate 38 kDa regions. Lane 1, 3, 5, and 7 (pH 6.0) are showed more dense band than Lane 2, 4, 6, and 8 (pH 7.2). Open circles represent protein bands highly increased under hypoxia and low pH condition with different media composition respectively (The arrow indicates the position of the region of 38 kDa antigen.) Proteins were visualized by Coomassie blue staining.
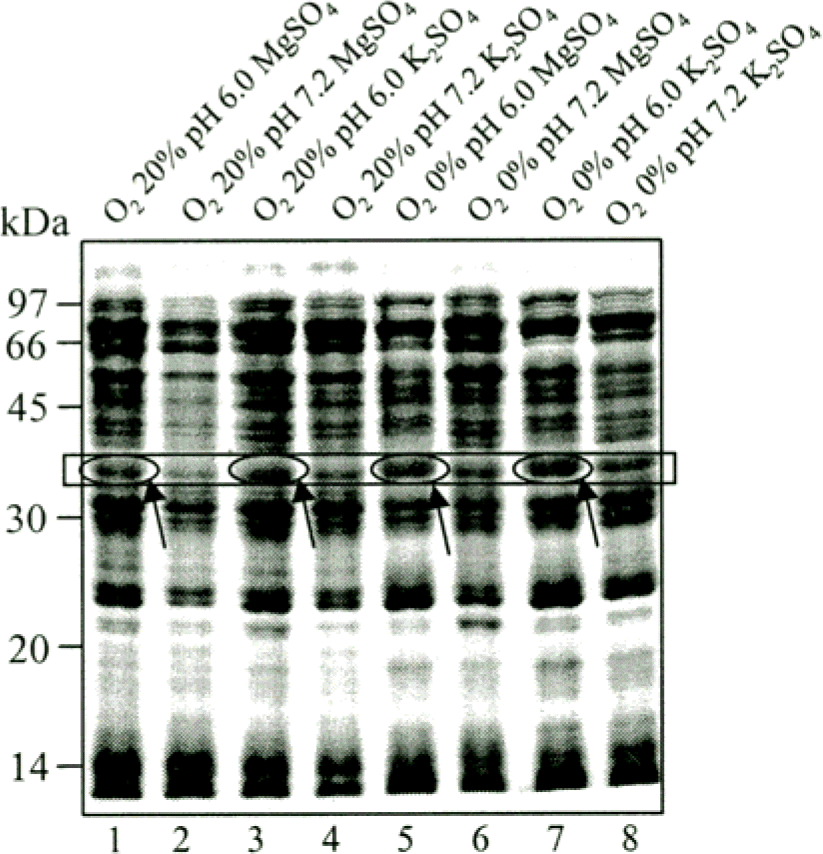
Figure 6.
2-DE analysis of the CF of M. tuberculosis H37Rv grown at 8 different conditions. M. tuberculosis were cultured in Sautons medium at 20% O2 (A) and at 0% O2 tension (B) with following the conditions. a, pH 6.0 Sauton medium with MgSO4; b. pH 7.2 Sauton medium with MgSO4; c. pH 6.0 Sauton medium with K2SO4; d. pH 7.2 Sauton medium with K2SO4. All condition samples were separated by isoelectric focusing (pH 4.7–5.9) and by 12% SDS-PAGE. The gels were stained with Coomassie blue.
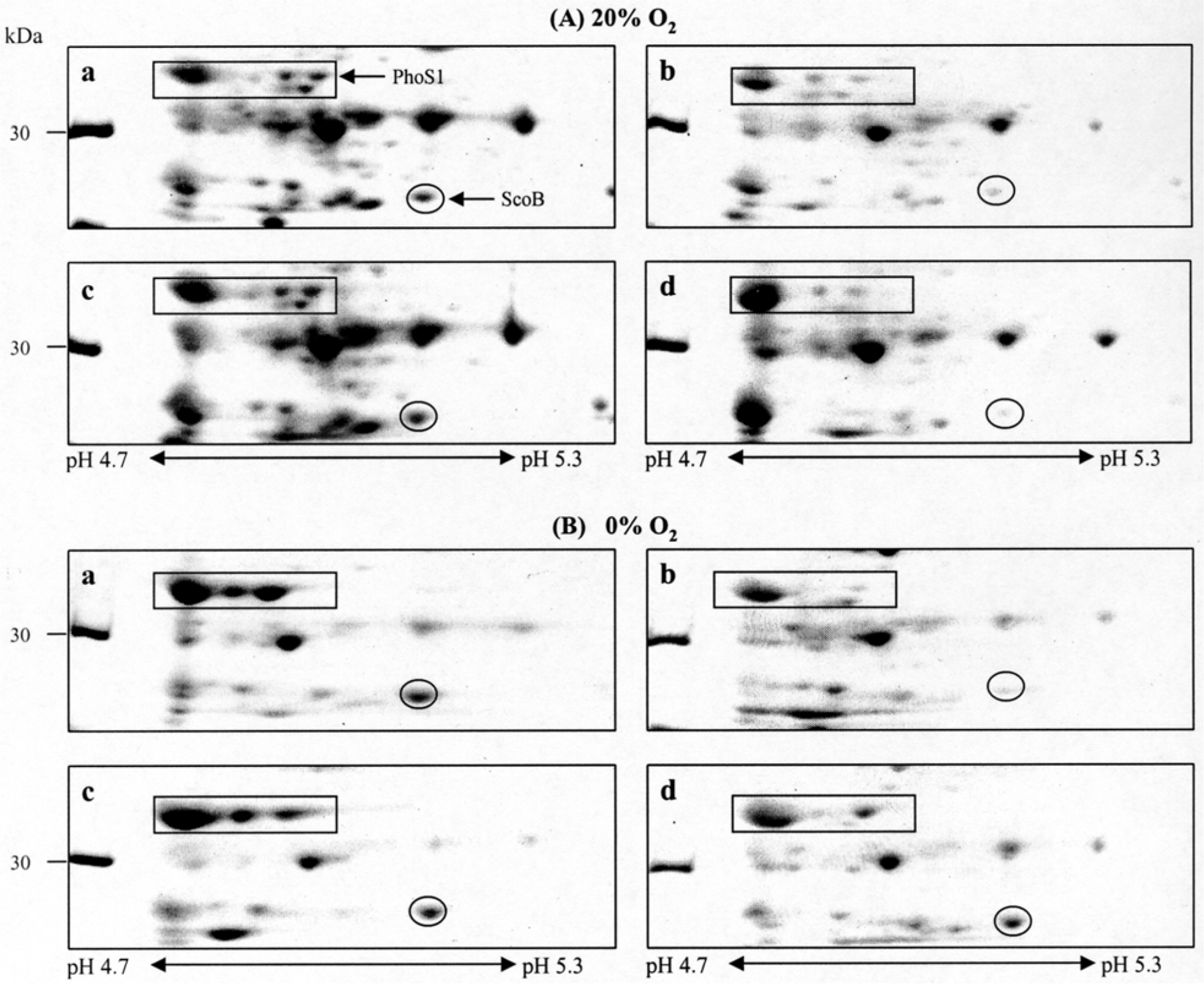
Table 1.
Culture condition and composition of Sauton media
| No. | pH | Sauton media | Culture condition |
|---|---|---|---|
| 1 | 6.0 | Sautona) | 20% O2 |
| 2 | 7.2 | Sautona) | 20% O2 |
| 3 | 6.0 | Modifiedb) | 20% O2 |
| 4 | 7.2 | Modifiedb) | 20% O2 |
| 5 | 6.0 | Sautona) | 0% O2 |
| 6 | 7.2 | Sautona) | 0% O2 |
| 7 | 6.0 | Modifiedb) | 0% or 13%∗ O2 |
| 8 | 7.2 | Modifiedb) | 0% O2 |
Table 2.
M. tuberculosis H37Rv proteins significantly induced by low-oxygen condition and/or low pH on 2-DE




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download