Abstract
Helicobacter pylori-infected gastric mucosa is characterized by infiltration of various inflammatory cells such as neutrophils and eosinophils. Although several mechanisms for neutrophil infiltration are well known, there has been little known the role of eotaxin, which is a potent chemoattractant for eosinophils, on the inflammatory process of H. pylori infection. The present study was to investigate the mechanisms of eotaxin expression in gastric epithelial cells stimulated with H. pylori vacuolating cytotoxin (VacA). Stimulation with VacA purified from VacA+ H. pylori slightly increased eotaxin expression in MKN-45 gastric epithelial cells. In contrast, the combined stimulation with VacA and IL-4 synergistically increased the eotaxin expression as determined by quantitative RT-PCR and ELISA. In MKN-45 cells transfected with an eotaxin promoter-luciferase reporter plasmid, costimulation with VacA and IL-4 induced more luciferase activity than either VacA or IL-4 alone did. However, such up-regulation was significantly decreased in the cells transfected with luciferase reporter plasmid bearing an eotaxin promoter which has a mutation at STAT6 binding site. These results suggest that the up-regulation of eotaxin in VacA-stimulated gastric epithelial cells may be synergistically facilitated by IL-4 via a STAT6-dependent mechanism.
References
1). 김정목, 강신재, 조수진, 정훈용, 오유경, 김영전. Bacteroides fragilis 장독소 자극을 받은 인체 장상피세포에 서의 cyclooxygenase-2의 발현. J Bacteriol Virol. 32:147–157. 2002.
2). 김정목, 정현채, 조양자, 송인성, 김정룡. Helicobacter pylori의 실험적 감염에 의하여 유발되는 인체 위상피세 포에서의 proinflammatory cytokine 유전자의 발현 양상 및 정량. 대한미생물학회지. 30:651–663. 1995.
3). Baek HY, Lim JW, Kim H, Kim JM, Kim JS, Jung HC, Kim KH. Oxidative stress-related proteome changes in Heli cobacter pylori-infected human gastric mucosa. Biochem J. 379(Pt 2):291–299. 2004.
4). Comtois SL, Gidley MD, Kelly DJ. Role of the thioredoxin system and the thiol-peroxidases Tpx and Bcp in mediating resistance to oxidative and nitrosative stress in Helicobacter pylori. Microbiology. 149:121–129. 2003.
5). Cover TL, Hanson PI, Heuser JE. Acid-induced dissociation of VacA, the Helicobacter pylori vacuolating cytotoxin, reveals its pattern of assembly. J Cell Biol. 138:759–769. 1997.
6). de Bernard M, Papini E, de Filippis V, Gottardi E, Telford J, Manetti R, Fontana A, Rappuoli R, Montecucco C. Low pH activates the vacuolating toxin of Helicobacter pylori, which becomes acid and pepsin resistant. J Biol Chem. 270:23937–23940. 1995.
7). Del Giudice G, Covacci A, Telford JL, Montecucco C, Rappuoli R. The design of vaccines against Helicobacter pylori and their development. Annu Rev Immunol. 19:523–563. 2001.
8). Forssmann U, Uguccioni M, Loetscher P, Dahinden CA, Langen H, Thelen M, Baggiolini M. Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR3, and acts like eotaxin on human eosinophil and basophil leukocytes. J Exp Med. 185:2171–2176. 1997.

9). Gebert B, Fischer W, Haas R. The Helicobacter pylori vacuolating cytotoxin: from cellular vacuolation to immunosuppressive activities. Rev Physiol Biochem Pharmacol. 152:205–220. 2004.
10). Go MF. Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 16(Suppl 1):3–15. 2002.
11). Hou J, Schindler U, Henzel WJ, Ho TC, Brasseur M, McKnight SL. An interleukin-4-induced transcription factor: IL-4 STAT. Science. 265:1701–1706. 1994.

12). Jedrzkiewicz S, Nakamura H, Silverman ES, Luster AD, Mansharamani N, In KH, Tamura G, Lilly CM. IL-1beta induces eotaxin gene transcription in A549 airway epithelial cells through NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 279:L1058–L1065. 2000.
13). Kikuchi T, Kato K, Ohara S, Sekine H, Arikawa T, Suzuki T, Noguchi K, Saito M, Saito Y, Nagura H, Toyota T, Shimosegawa T. The relationship between persistent secretion of RANTES and residual infiltration of eosinophils and memory T lymphocytes after Helicobacter pylori eradication. J Pathol. 192:243–250. 2000.
14). Kim JM, Jung HY, Lee JY, Youn J, Lee CH, Kim KH. Mitogen-activated protein kinase and activator protein-1 dependent signals are essential for Bacteroides fragilis enterotoxin-induced enteritis. Eur J Immunol. 35:2648–2657. 2005.
15). Kim JM, Kim JS, Jung HC, Song IS, Kim CY. Virulence factors of Helicobacter pylori in Korean strains do not influence proinflammatory cytokine gene expression and apoptosis in human gastric epithelial cells, nor do these factors influence clinical outcome. J Gastroenterol. 35:898–906. 2000.
16). Kim JM, Kim JS, Jung HC, Oh YK, Chung HY, Lee CH, Song IS. Helicobacter pylori infection activates the NF-κB signaling pathway to induce iNOS and protect human gastric epithelial cells from apoptosis. Am J Physiol – Gastrointest Liver. 285:G1171–1180. 2003.
17). Kim JM, Kim JS, Jung HC, Song IS, Kim CY. Up-regulation of inducible nitric oxide synthase and nitric oxide in Helicobacter pylori-infected human gastric epithelial cells: possible role of interferon-γ in polarized nitric oxide secretion. Helicobacter. 7:116–128. 2002.
18). Kitaura M, Suzuki N, Imai T, Takagi S, Suzuki R, Nakajima T, Hirai K, Nomiyama H, Yoshie O. Molecular cloning of a novel human CC chemokine (Eotaxin-3) that is a functional ligand of CC chemokine receptor 3. J Biol Chem. 274:27975–27980. 1999.

19). Komiya A, Nagase H, Yamada H, Sekiya T, Yamaguchi M, Sano Y, Hanai N, Furuya A, Ohta K, Matsushima K, Yoshie O, Yamamoto K, Hirai K. Concerted expression of eotaxin-1, eotaxin-2, and eotaxin-3 in human bronchial epithelial cells. Cell Immunol. 225:91–100. 2003.

20). Matsukura S, Stellato C, Georas SN, Casolaro V, Plitt JR, Miura K, Kurosawa S, Schindler U, Schleimer RP. Interleukin-13 upregulates eotaxin expression in airway epithelial cells by a STAT6-dependent mechanism. Am J Respir Cell Mol Biol. 24:755–761. 2001.

21). Matsukura S, Stellato C, Plitt JR, Bickel C, Miura K, Georas SN, Casolaro V, Schleimer RP. Activation of eotaxin gene transcription by NF-kappa B and STAT6 in human airway epithelial cells. J Immunol. 163:6876–6883. 1999.
22). McGovern TW, Talley NJ, Kephart GM, Carpenter HA, Gleich GJ. Eosinophil infiltration and degranulation in Helicobacter pylori-associated chronic gastritis. Dig Dis Sci. 36:435–440. 1991.
23). Mikita T, Campbell D, Wu P, Williamson K, Schindler U. Requirements for interleukin-4-induced gene expression and functional characterization of Stat6. Mol Cell Biol. 16:5811–5820. 1996.

24). Molinari M, Salio M, Galli C, Norais N, Rappuoli R, Lanzavecchia A, Montecucco C. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J Exp Med. 187:135–140. 1998.
25). Montecucco C, Rappuoli R. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat Rev Mol Cell Biol. 2:457–466. 2001.
26). Nakamura H, Haley KJ, Nakamura T, Luster AD, Lilly CM. Differential regulation of eotaxin expression by TNF-alpha and PMA in human monocytic U-937 cells. Am J Physiol. 275:L601–L610. 1998.
27). Orsini B, Ottanelli B, Amedei A, Surrenti E, Capanni M, Del Prete G, Amorosi A, Milani S, D'Elios MM, Surrenti C. Helicobacter pylori cag pathogenicity island is associated with reduced expression of interleukin-4 (IL-4) mRNA and modulation of the IL-4delta2 mRNA isoform in human gastric mucosa. Infect Immun. 71:6664–6667. 2003.
28). Papini E, Zoratti M, Cover TL. In search of the Helicobacter pylori VacA mechanism of action. Toxicon. 39:1757–1767. 2001.
29). Perez-Perez GI, Peek RM, Legath AJ, Heine PR, Graff LB. The role of CagA status in gastric and extragastric complications of Helicobacter pylori. J Physiol Pharmacol. 50:833–845. 1999.
30). Pesci EC, Pickett CL. Genetic organization and enzymatic activity of a superoxide dismutase from the microaerophilic human pathogen, Helicobacter pylori. Gene. 143:111–116. 1994.
31). Pfitzner E, Kliem S, Baus D, Litterst CM. The role of STATs in inflammation and inflammatory diseases. Curr Pharm Des. 10:2839–2850. 2004.
32). Prinz C, Hafsi N, Voland P. Helicobacter pylori virulence factors and the host immune response: implications for therapeutic vaccination. Trends Microbiol. 11:134–138. 2003.
33). Reyrat JM, Pelicic V, Papini E, Montecucco C, Rappuoli R, Telford JL. Towards deciphering the Helicobacter pylori cytotoxin. Mol Microbiol. 34:197–204. 1999.
34). Slungaard A, Vercellotti GM, Walker G, Nelson RD, Jacob HS. Tumor necrosis factor alpha/cachectin stimulates eosinophil oxidant production and toxicity towards human endothelium. J Exp Med. 171:2025–2041. 1990.

35). Supajatura V, Ushio H, Wada A, Yahiro K, Okumura K, Ogawa H, Hirayama T, Ra C. Cutting edge. VacA, a vacuolating cytotoxin of Helicobacter pylori, directly activates mast cells for migration and production of proinflammatory cytokines. J Immunol. 168:2603–2607. 2002.
36). van Vliet AH, Kuipers EJ, Stoof J, Poppelaars SW, Kusters JG. Acid-responsive gene induction of ammonia-producing enzymes in Helicobacter pylori is mediated via a metal-responsive repressor cascade. Infect Immun. 72:766–773. 2004.
37). Wada A, Yamasaki E, Hirayama T. Helicobacter pylori vacuolating cytotoxin, VacA, is responsible for gastric ulceration. J Biochem. 136:741–746. 2004.
38). Walsh GM. Eosinophil granule proteins and their role in disease. Curr Opin Hematol. 8:28–33. 2001.

39). Whitney AE, Guarner J, Hutwagner L, Gold BD. Helicobacter pylori gastritis in children and adults: comparative histopathologic study. Ann Diagn Pathol. 4:279–285. 2000.
40). Williams TJ, Jose PJ. Role of eotaxin and related CC chemokines in allergy and asthma. Chem Immunol. 78:166–177. 2000.

41). Wong CK, Zhang J, Ip WK, Lam CW. Intracellular signal transduction in eosinophils and its clinical significance. Immunopharmacol Immunotoxicol. 24:165–186. 2002.

42). Wu W, Chen Y, Hazen SL. Eosinophil peroxidase nitrates protein tyrosyl residues. Implications for oxidative damage by nitrating intermediates in eosinophilic inflammatory disorders. J Biol Chem. 274:25933–25944. 1999.
Figure 1.
Purification of H. pylori vacuolating cytotoxin. (A) Immunoblot analysis of VacA preparations electrophoresed on a 10% acrylamide gel, transferred to nitrocellulose, and reacted with goat anti-VacA antibody. (B) Vacuolation in HeLa cells treated with H. pylori vacuolating cytotoxin. HeLa cells in a 96 well plate were treated with H. pylori vacuolating cytotoxin (20 μg/ml) for 24 h. Vacuolation was observed by an inverted microscopy (× 300).
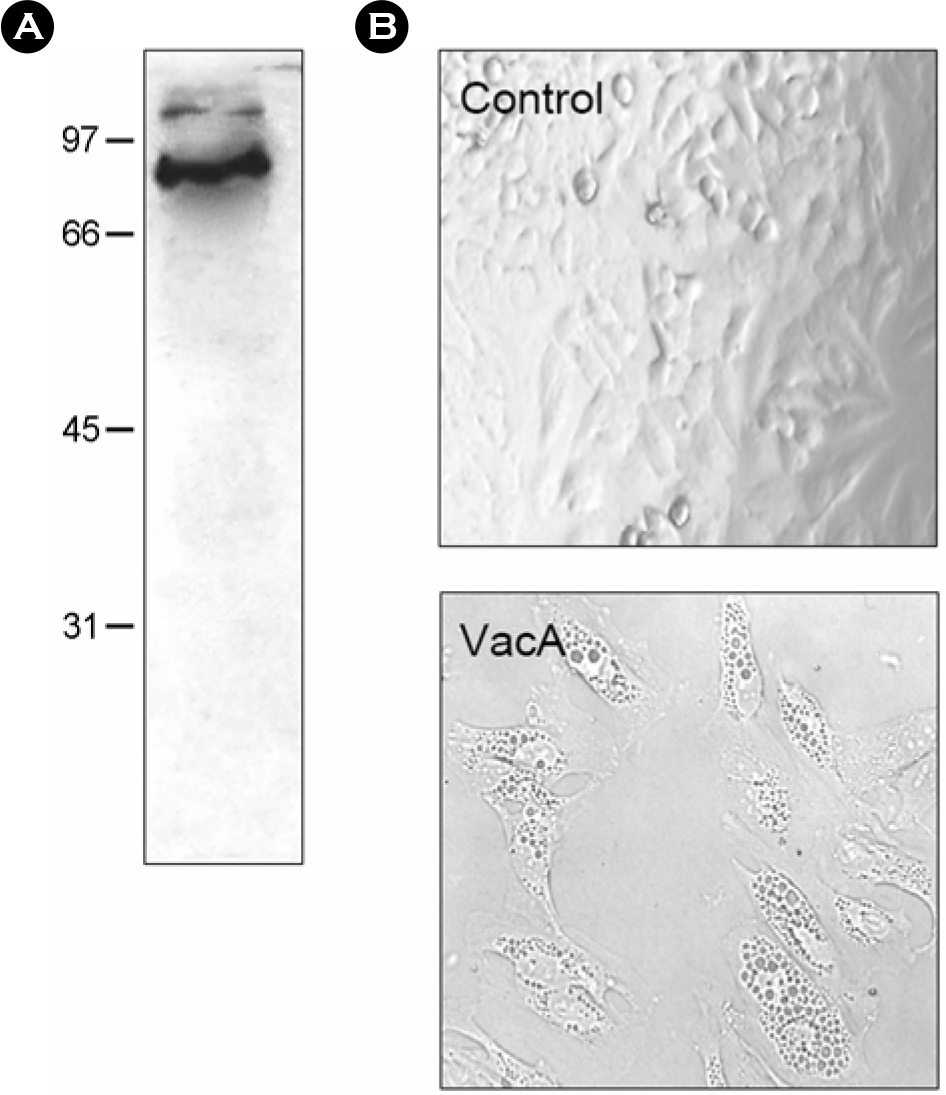
Figure 2.
RT-PCR for eotaxin expression in MKN-45 cells treated with H. pylori vacuolating cytotoxin and/or IL-4. Confluent monolayers of MKN-45 cells were treated with H. pylori vacuolating cytotoxin (5 μg/ml) and/or IL-4 (50 ng/ml) for the indicated hours, after which cellular RNA was extracted. In this experiment, tissue culture medium was also supplemented with 5 mM NH4Cl. The (+) and (–) represent positive and negative controls, respectively.
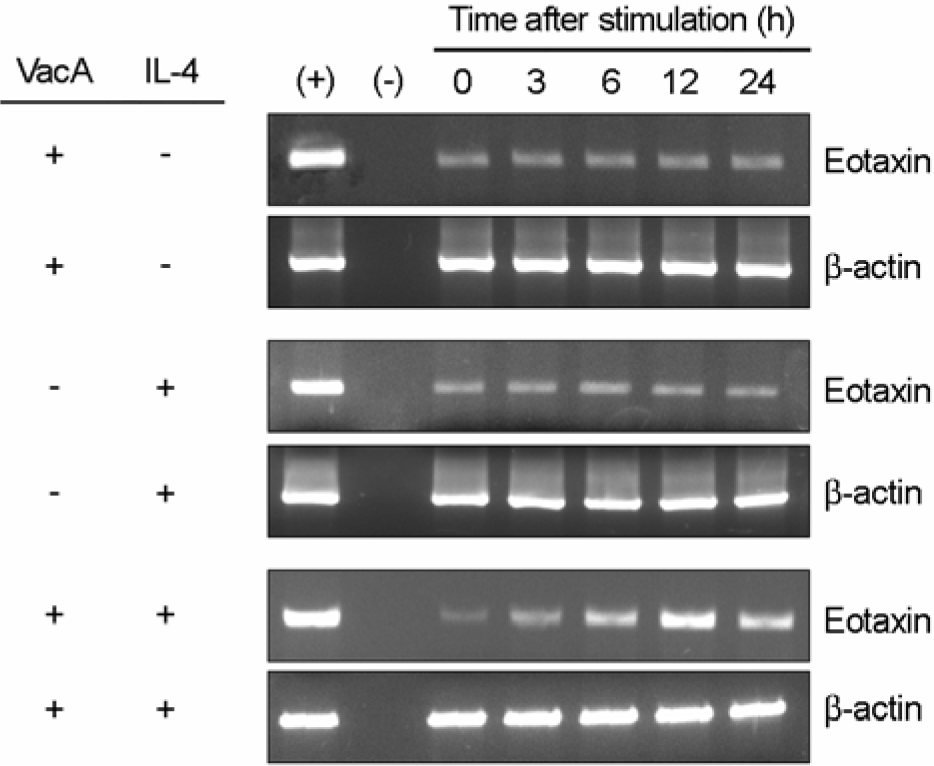
Figure 3.
Quantification of eotaxin mRNA molecules in MKN-5 cells treated with H. pylori vacuolating cytotoxin and/or IL-4. Confluent monolayers of MKN-45 cells were treated with H. pylori vacuolating cytotoxin (5 μg/ml, VacA) and/or IL-4 (50 ng/ml) for the indicated hours, after which cellular RNA was extracted. Quantification of eotaxin and β-actin mRNA molecules was performed as described by Materials and Methods. In this experiment, tissue culture medium was also supplemented with 5 mM NH4Cl. The values are expressed as the mean of five different experiments.
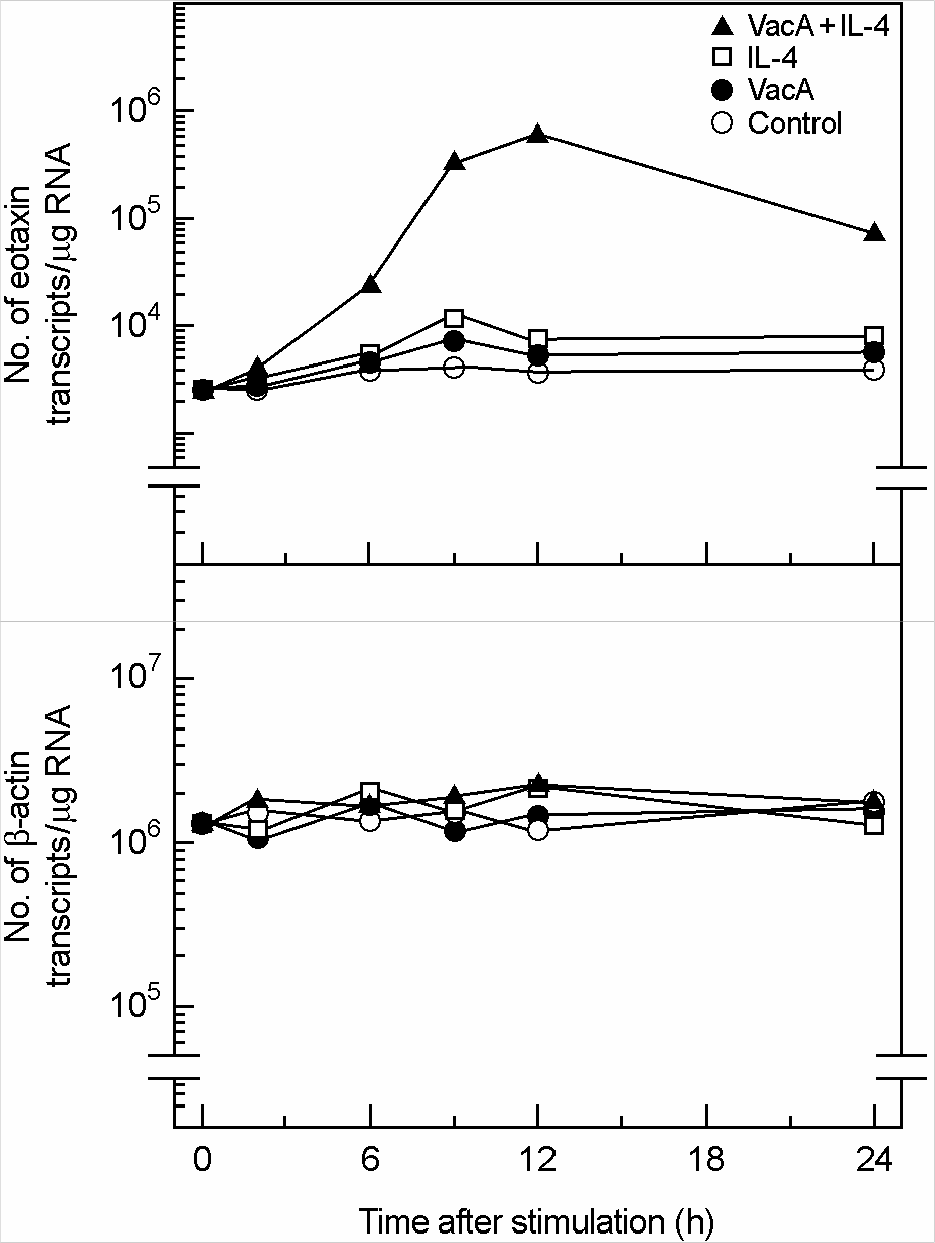
Figure 4.
Eotaxin production in MKN-45 cells treated with H. pylori vacuolating cytotoxin and/or IL-4. Culture supernatants of MKN-45 cells were collected 24 h after stimulation with H. pylori vacuolating cytotoxin (5 μg/ml, VacA) and/or IL-4 (50 ng/ml). Tissue culture medium was also supplemented with 5 mM NH4Cl. Protein levels of eotaxin in culture supernatants were determined by ELISA. Data are mean ± SEM (n=5). ∗p<0.05 versus MKN-45 cells in medium without VacA or IL-4. ∗∗p<0.01 versus MKN-45 cells in medium with VacA alone or IL-4 alone.
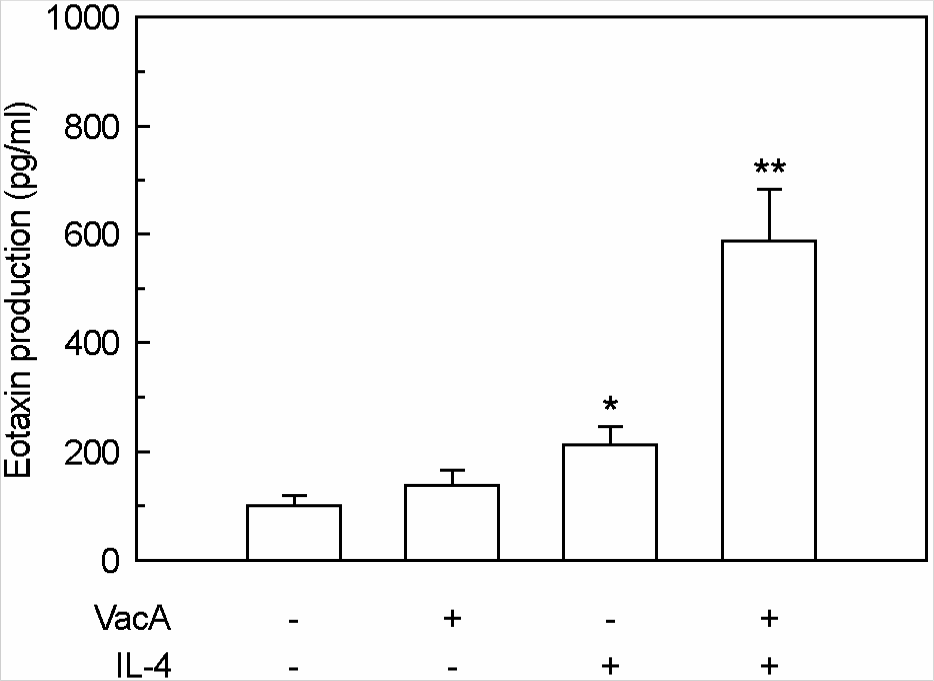
Figure 5.
The effects of live H. pylori on the eotaxin production in MKN-45 cells treated with IL-4. MKN-45 cells were incubated with IL-4 (50 ng/ml) in the presence of live H. pylori or purified VacA (5 μg/ml). Tissue culture medium was also supplemented with 5 mM NH4Cl. After 24 h of incubation, the levels of eotaxin protein were measured by ELISA. Results are expressed as the mean ± SEM of five experiments. ∗p<0.05 versus MKN-45 cells in medium with IL-4 alone.
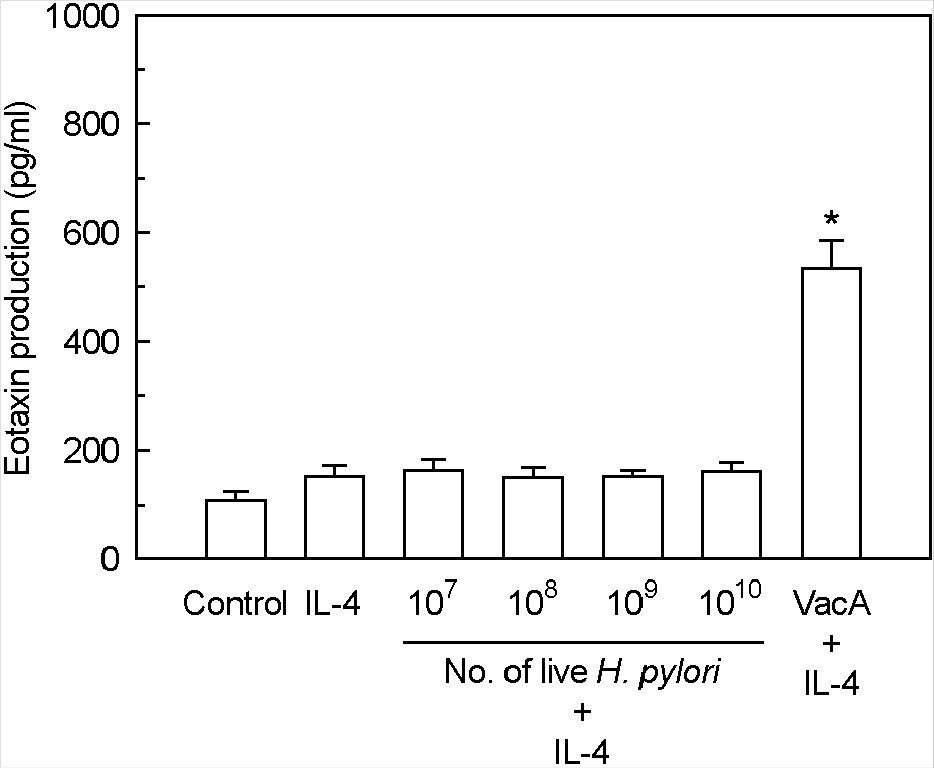
Figure 6.
Eotaxin production in IL-4-stimulated MKN-45 cells after incubation with acidified H. pylori vacuolating cytotoxin in the presence or absence of ammonium chloride. MKN-45 cells were incubated with IL-4 (50 ng/ml) in the presence of purified VacA (either acid pH or neutral pH, 5 μg/ml), or in the presence of buffer controls. Tissue culture medium was also either supplemented with 5 mM NH4Cl or not supplemented. After 24 h of incubation, eotaxin production was measured by ELISA. Results are expressed as the mean ± SEM of five experiments. ∗p<0.05 versus MKN-45 cells in medium without NH4Cl.
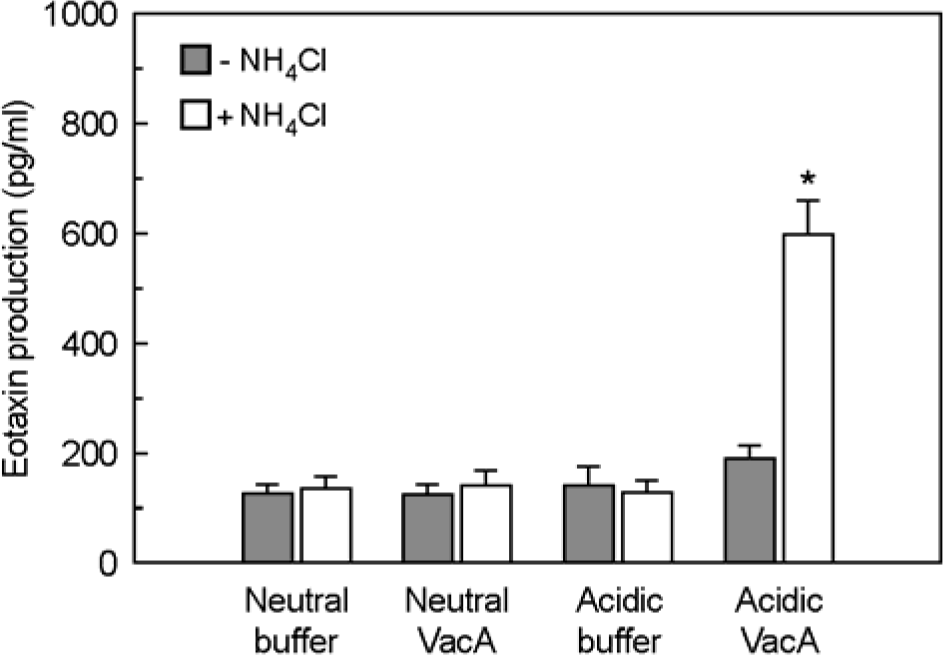
Figure 7.
Activation of eotaxin reporter gene in MKN-45 cells stimulated with H. pylori vacuolating cytotoxin and IL-4. MKN-45 cells were transfected with pEotaxin-luciferase transcriptional reporter (pEotx.1363). Forty-eight hours after transfection, cells were stimulated with the indicated concentrations of H. pylori vacuolating cytotoxin (VacA) and 50 ng/ml of IL-4 for 6 h. Tissue culture medium was also supplemented with 5 mM NH4Cl. Data are the mean fold induction in luciferase activity relative to unstimulated control. Values are the mean ± SEM of five experiments.
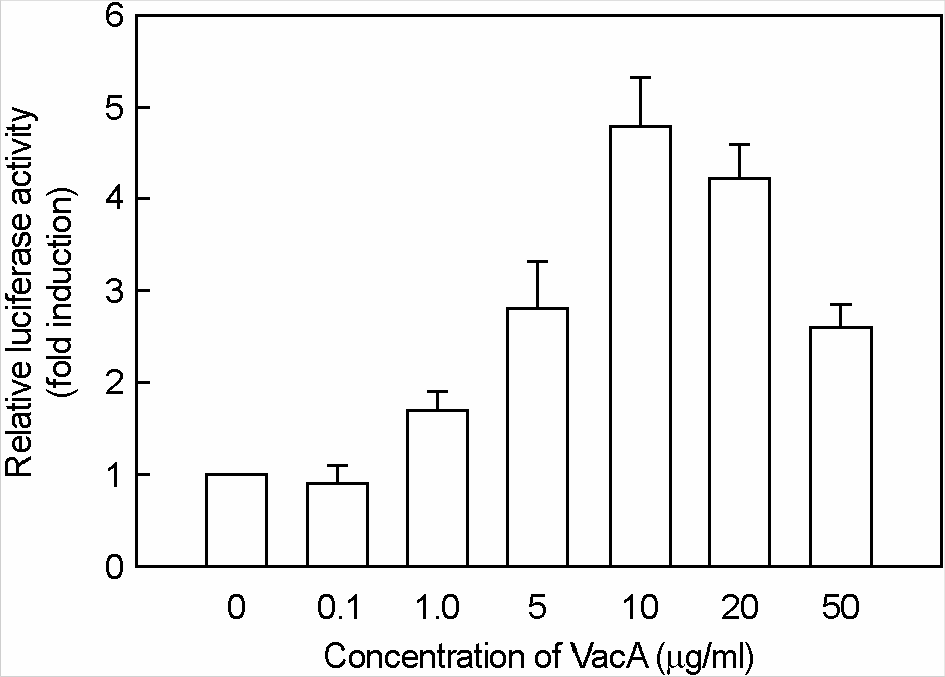
Figure 8.
Activation of the eotaxin promoter by STAT6. MKN-45 cells were transfected with either a wild-type eotaxin promotor luciferase reporter plasmid (pEotx.1363) or a plasmid mutated at binding site for STAT6 (pEotx.M1). Forty-eight hours after transfection, cells were incubated with or without VacA (5 μg/ml) and/or IL-4 (50 ng/ml) for 6 h and then harvested. Tissue culture medium was also supplemented with 5 mM NH4Cl. The luciferase activity normalized to protein concentration and calculated as the fold induction compared with the control vector. The data are presented as the mean ± SEM of five independent experiments. ∗, p<0.05 compared with pEotx.1363
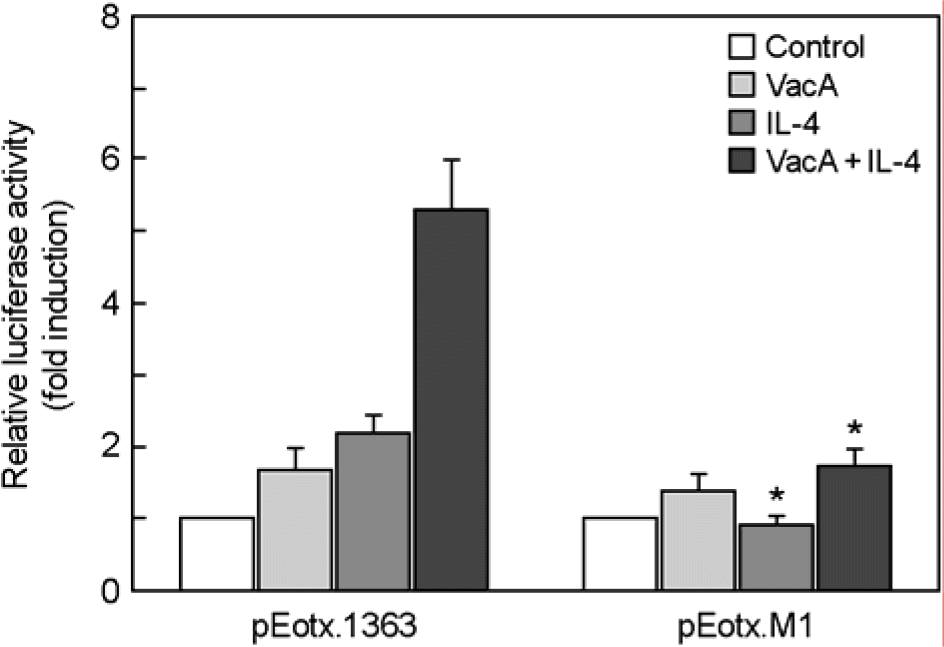
Table 1.
Sequences of oligonucleotide primers for RT-PCR analysis of human eotaxin and β-actin




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download