Abstract
Neuroendocrine tumor (NET) of the major duodenal papilla is a rare occurrence. However, that of the minor duodenal papilla is even rarer. To date, only a few cases have been reported. Herein, we present a rare case of NETs detected at the major and minor duodenal papilla synchronously, which were successfully treated with endoscopic papillectomy without procedure-related complication. To the best of our knowledge, this is the first report of this kind in the world. Photomicrograph of the biopsy specimen stained im-munohistochemically for synaptophysin showed a positive reaction of tumor cells. All resection margins were negative. Further experience with more cases will be needed to establish the exact indication of endoscopic papillectomy for duodenal papillary NETs.
References
1. Hatzitheoklitos E, Büchler MW, Friess H, et al. Carcinoid of the ampulla of Vater. Clinical characteristics and morphologic features. Cancer. 1994; 73:1580–1588.

2. Pyun DK, Moon G, Han J, et al. A carcinoid tumor of the ampulla of Vater treated by endoscopic snare papillectomy. Korean J Intern Med. 2004; 19:257–260.

3. Han J, Lee SK, Park DH, et al. Treatment outcome after endoscopic papillectomy of tumors of the major duodenal papilla. Korean J Gastroenterol. 2005; 46:110–119.
4. Will U, Müller AK, Fueldner F, Wanzar I, Meyer F. Endoscopic papillectomy: data of a prospective observational study. World J Gastroenterol. 2013; 19:4316–4324.

5. Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Moriyasu F. Endoscopic resection of carcinoid of the minor duodenal papilla. World J Gastroenterol. 2007; 13:3763–3764.

6. Albores-Saavedra J, Hart A, Chablé-Montero F, Henson DE. Carcinoids and high-grade neuroendocrine carcinomas of the ampulla of vater: a comparative analysis of 139 cases from the surveillance, epidemiology, and end results program-a population based study. Arch Pathol Lab Med. 2010; 134:1692–1696.

7. Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015; 50:58–64.

8. Randle RW, Ahmed S, Newman NA, Clark CJ. Clinical outcomes for neuroendocrine tumors of the duodenum and ampulla of Vater: a population-based study. J Gastrointest Surg. 2014; 18:354–362.
9. Carter JT, Grenert JP, Rubenstein L, Stewart L, Way LW. Neuroendocrine tumors of the ampulla of Vater: biological behavior and surgical management. Arch Surg. 2009; 144:527–531.
10. Clements WM, Martin SP, Stemmerman G, Lowy AM. Ampullary carcinoid tumors: Rationale for an aggressive surgical approach. J Gastrointest Surg. 2003; 7:773–776.

11. Dumitrascu T, Dima S, Herlea V, Tomulescu V, Ionescu M, Popescu I. Neuroendocrine tumours of the ampulla of Vater: clinicopathological features, surgical approach and assessment of prognosis. Langenbecks Arch Surg. 2012; 397:933–943.

12. Rattner DW, Fernandez-del Castillo C, Brugge WR, Warshaw AL. Defining the criteria for local resection of ampullary neoplasms. Arch Surg. 1996; 131:366–371.

13. Salmi S, Ezzedine S, Vitton V, et al. Can papillary carcinomas be treated by endoscopic ampullectomy? Surg Endosc. 2012; 26:920–925.

14. Virgilio E, La Gumina G, Tozzi F, Marrero YC, Ziparo V, Cavallini M. Neuroendocrine tumor of the minor duodenal papilla: an unusual cause of pancreaticoduodenectomy. Am Surg. 2016; 82:1145–1148.

15. Noda Y, Watanabe H, lwafuchi M, et al. Carcinoids and endocrine cell micronests of the minor and major duodenal papillae. Their incidence and characteristics. Cancer. 1992; 70:1825–1833.

16. Kim YG, Kim TN, Kim KO. Carcinoid tumor of the minor papilla in complete pancreas divisum presenting as recurrent abdominal pain. BMC Gastroenterol. 2010; 10:17.

17. Makhlouf HR, Burke AP, Sobin LH. Carcinoid tumors of the ampulla of Vater: a comparison with duodenal carcinoid tumors. Cancer. 1999; 85:1241–1249.
18. Maruyama T, Shirai Y, Sakata J, Wakai T, Iwafuchi M, Hatakeyama K. A 1.3-cm carcinoid tumor of the minor duodenal papilla with superior mesenteric lymph node metastases. Surgery. 2012; 151:340–341.

19. Burke AP, Federspiel BH, Sobin LH, Shekitka KM, Helwig EB. Carcinoids of the duodenum. A histologic and immunohistochemical study of 65 tumors. Am J Surg Pathol. 1989; 13:828–837.
Fig. 1.
MRCP image showing clinically normal biliary tree and pancreatic duct. MRCP, magnetic resonance cholangiopancreatography.

Fig. 2.
Side-viewing duodenoscopic findings for minor duodenal papilla. (A) Endoscopy showing an enlarged tumor at the minor duodenal papilla, with shallow ulcers and ectatic vessels at the top of the tumor. (B) Gross finding of the resected specimen after endoscopic snare minor papillectomy.
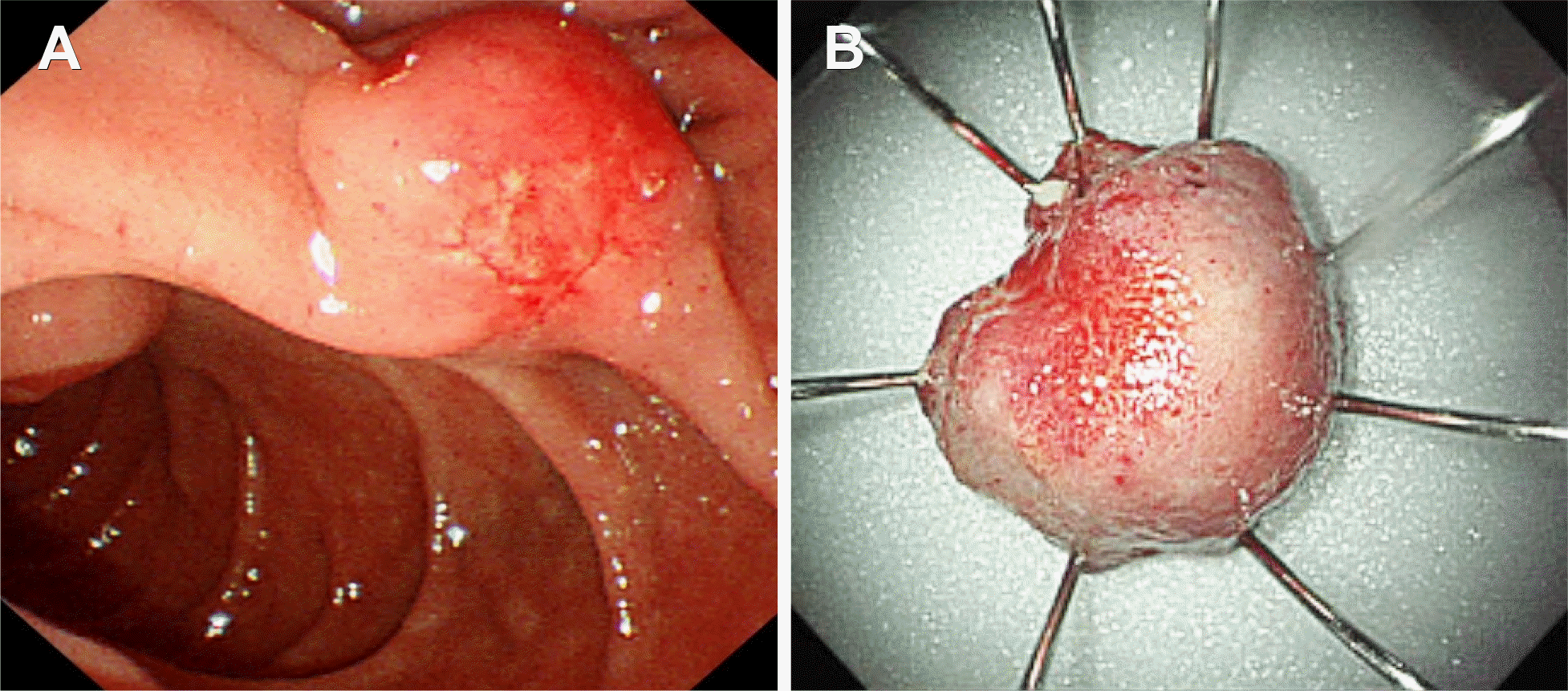
Fig. 3.
Pathologic findings of a neuroendocrine tumor of the minor duodenal papilla. (A) On low-power view, glandular proliferation of tumor cells was seen in mucosa and submucosa. Lymphovascular and perineural invasion was not observed (H&E, ×40). (B) On high-power view, tumor cells were monomorphic and uniform-sized, showing a trabecular, rosette pattern with small round cells featuring small round nuclei and pink-to-pale cytoplasm (H&E, ×400). (C) Tumor cells showing positivity for Ki-67 (Ki-67 stain, ×200). (D) Tumor cells were reactive to synaptophysin immunohistochemistry, evidence of neuroendocrine neoplasm (synaptophysin stain, ×200).
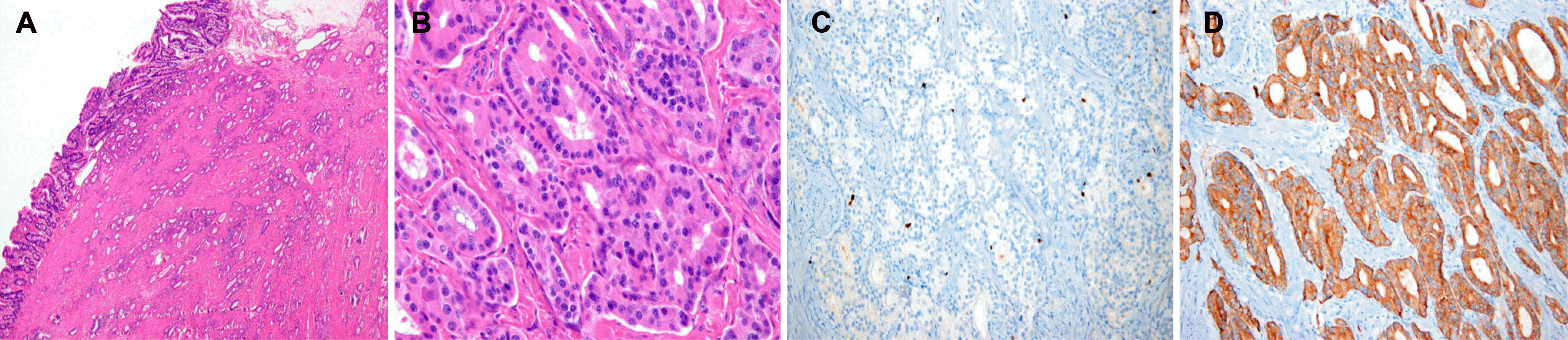
Fig. 4.
Side-viewing duodenoscopic findings for major duodenal papilla. (A) Endoscopy showing a rather prominent major duodenal papilla, but with preserved configuration. There were hyperemic granular changes and ectatic vessels. (B) Gross finding of the resected specimen after endoscopic snare major papillectomy.
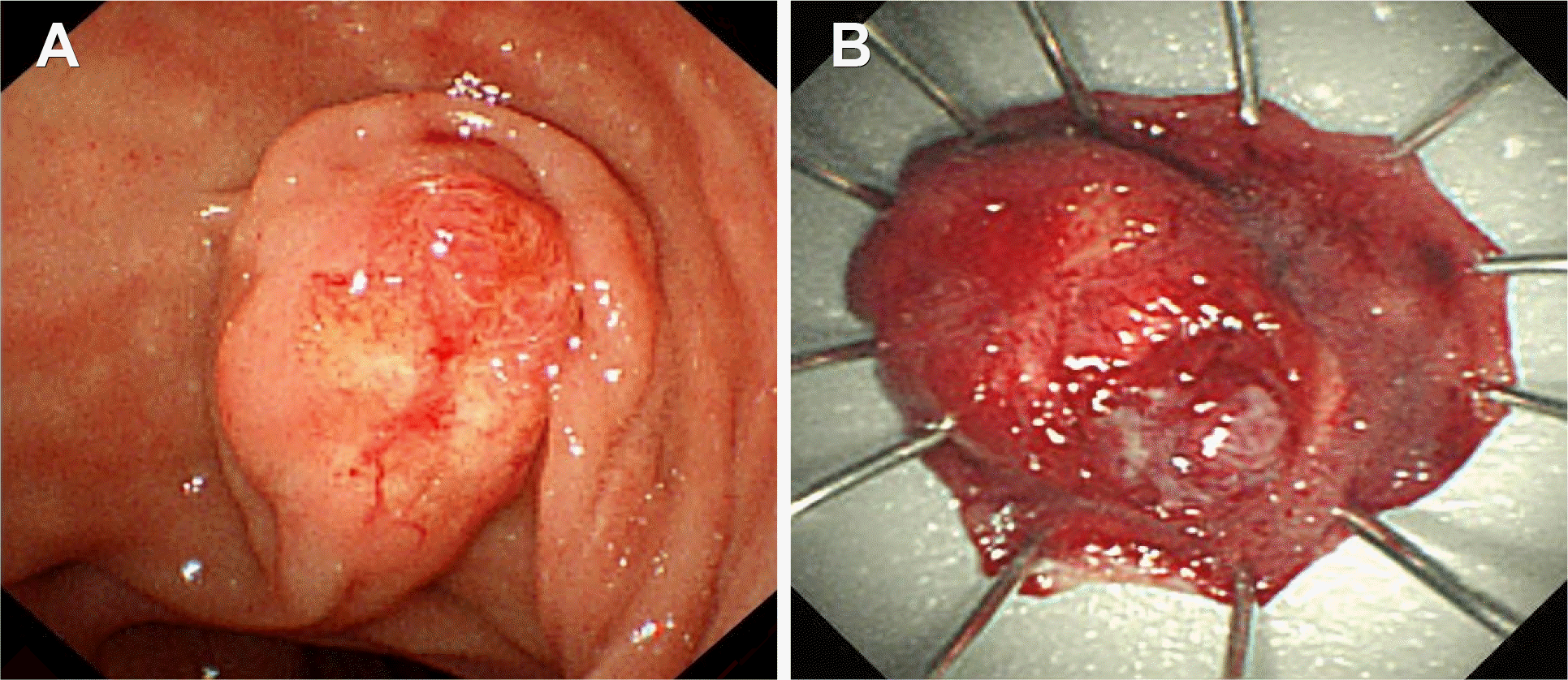
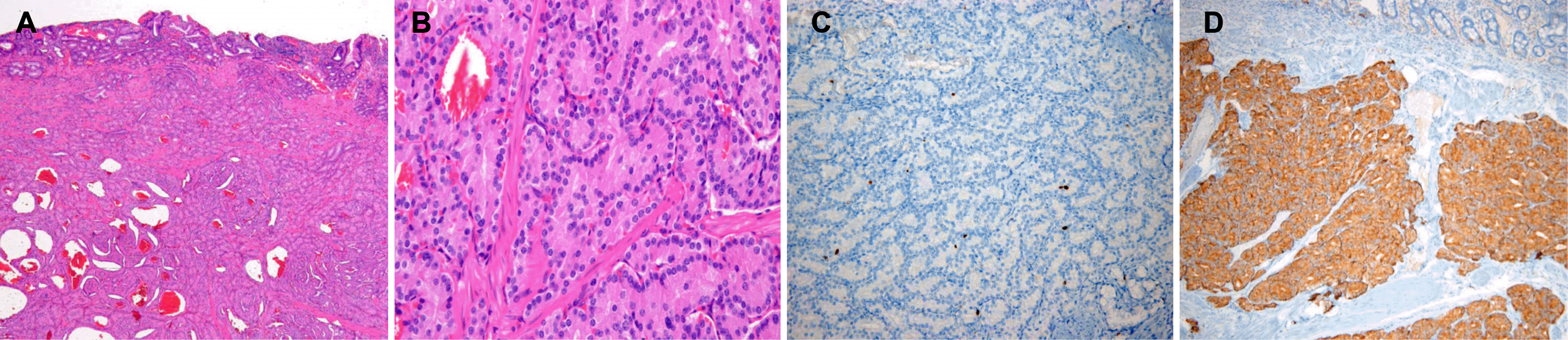
Fig. 5.
Pathologic findings of a neuroendocrine tumor of the major duodenal papilla. (A) On low-power view, tumor cells were limited to submucosa. Lymphovascular and perineural invasion was not seen (H&E, ×40). (B) On high-power view, tumor cells were monomorphic, uniform-sized, and showed a trabecular, rosette pattern with small round cells featuring small round nuclei and pink-to-pale cytoplasm (H&E,×400). (C) Tumor cells showing positive reaction for Ki-67 (Ki-67 stain, ×200). (D) Tumor cells were reactive to synaptophysin immunohistochemistry, evidence of neuroendocrine neoplasm (synaptophysin stain, ×100).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download