References
1. Wiedenmann B, Huttner WB. Synaptophysin and chromogra-nins/secretogranins–widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989; 58:95–121.
2. Wiedenmann B, Waldherr R, Buhr H, Hille A, Rosa P, Huttner WB. Identification of gastroenteropancreatic neuroendocrine cells in normal and neoplastic human tissue with antibodies against synaptophysin, chromogranin A, secretogranin I (chromogranin B), and secretogranin II. Gastroenterology. 1988; 95:1364–1374.

3. Helpap B, Köllermann J. Immunohistochemical analysis of the proliferative activity of neuroendocrine tumors from various organs. Are there indications for a neuroendocrine tumor- carcinoma sequence? Virchows Arch. 2001; 438:86–91.

4. Klimstra DS, Arnold R, Capella C, et al. Neuroendocrine neoplasm of the pancreas. Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. 4th ed.Lyon: International Agency for Research on Cancer;2010. p. 322–326.
5. Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010; 39:707–712.
6. Ehehalt F, Saeger HD, Schmidt CM, Grützmann R. Neuroendocrine tumors of the pancreas. Oncologist. 2009; 14:456–467.

7. Burke AP, Sobin LH, Federspiel BH, Shekitka KM, Helwig EB. Carcinoid tumors of the duodenum. A clinicopathologic study of 99 cases. Arch Pathol Lab Med. 1990; 114:700–704.
8. Gastrointestinal Pathology Study Group of Korean Society of Pathologists. Cho MY, Kim JM, Sohn JH, et al. Current trends of the incidence and pathological diagnosis of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in Korea 2000–2009: multicenter study. Cancer Res Treat. 2012; 44:157–165.

9. Kwaan MR, Goldberg JE, Bleday R. Rectal carcinoid tumors: review of results after endoscopic and surgical therapy. Arch Surg. 2008; 143:471–475.
10. Mani S, Modlin IM, Ballantyne G, Ahlman H, West B. Carcinoids of the rectum. J Am Coll Surg. 1994; 179:231–248.
11. Naunheim KS, Zeitels J, Kaplan EL, et al. Rectal carcinoid tumors–treatment and prognosis. Surgery. 1983; 94:670–676.
12. Schindl M, Niederle B, Häfner M, et al. Stage-dependent therapy of rectal carcinoid tumors. World J Surg. 1998; 22:628–633.

13. Tsai BM, Finne CO, Nordenstam JF, et al. Transanal endoscopic microsurgery resection of rectal tumors: outcomes and recommendations. Dis Colon Rectum. 2010; 53:16–23.

14. Ballantyne GH, Savoca PE, Flannery JT, Ahlman MH, Modlin IM. Incidence and mortality of carcinoids of the colon. Data from the Connecticut Tumor Registry. Cancer. 1992; 69:2400–2405.

15. Yantiss RK, Odze RD, Farraye FA, Rosenberg AE. Solitary versus multiple carcinoid tumors of the ileum: a clinical and pathologic review of 68 cases. Am J Surg Pathol. 2003; 27:811–817.
16. Gulec SA, Mountcastle TS, Frey D, et al. Cytoreductive surgery in patients with advanced-stage carcinoid tumors. Am Surg. 2002; 68:667–671.
17. Kunz PL, Kuo T, Zahn JM, et al. A phase II study of capecitabine, oxaliplatin, and bevacizumab for metastatic or unresectable neuroendocrine tumors (abstract 4104). J Clin Oncol. 2010; 28(15 Suppl):326.
Fig. 1.
Abdominal CT showing homogeneously enhancing mass in the distal ileum with multiple lymph node enlargement, small bowel mesentery and mesenteric haziness (arrow).
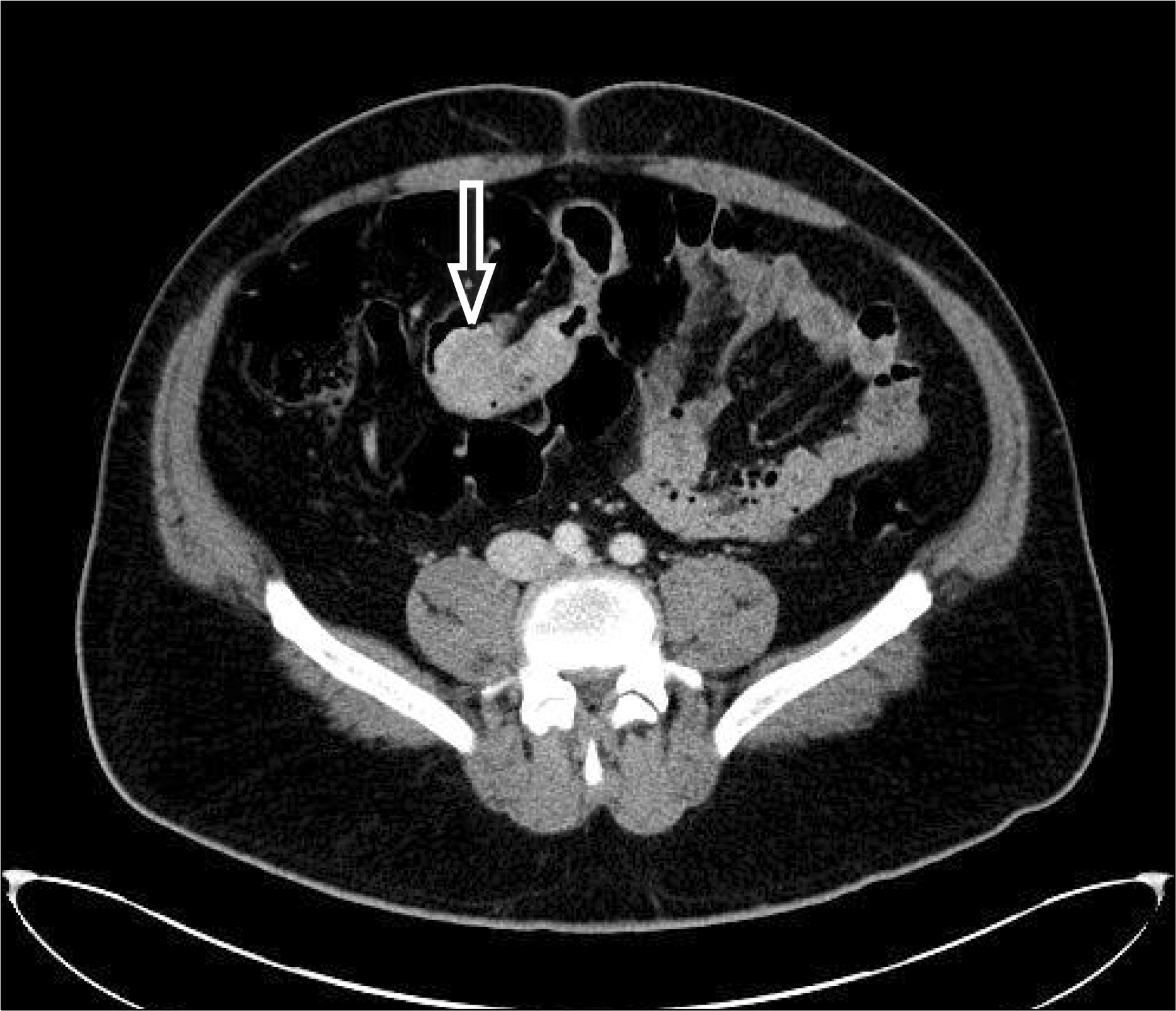
Fig. 3.
PET-CT showing hypermetabolic malignancy in the ileum with multiple regional lymph node metastasis in the small bowel mesentery (arrow).
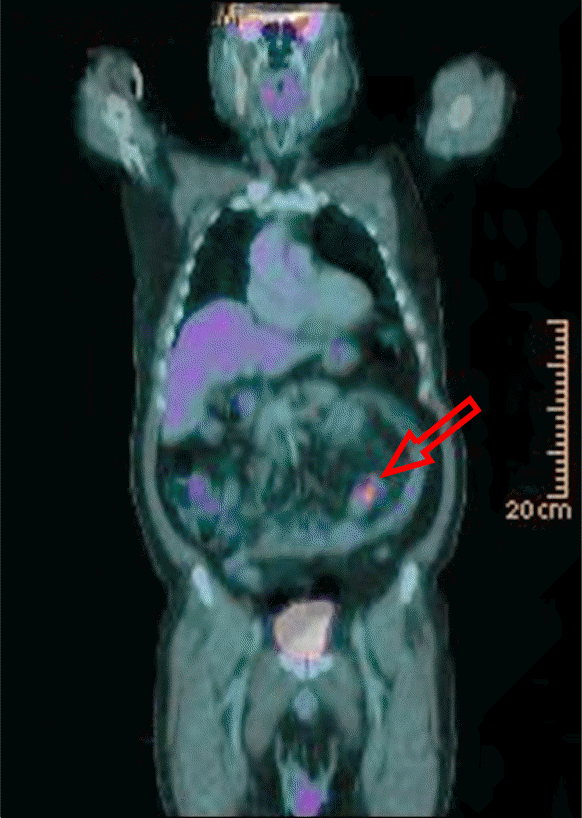
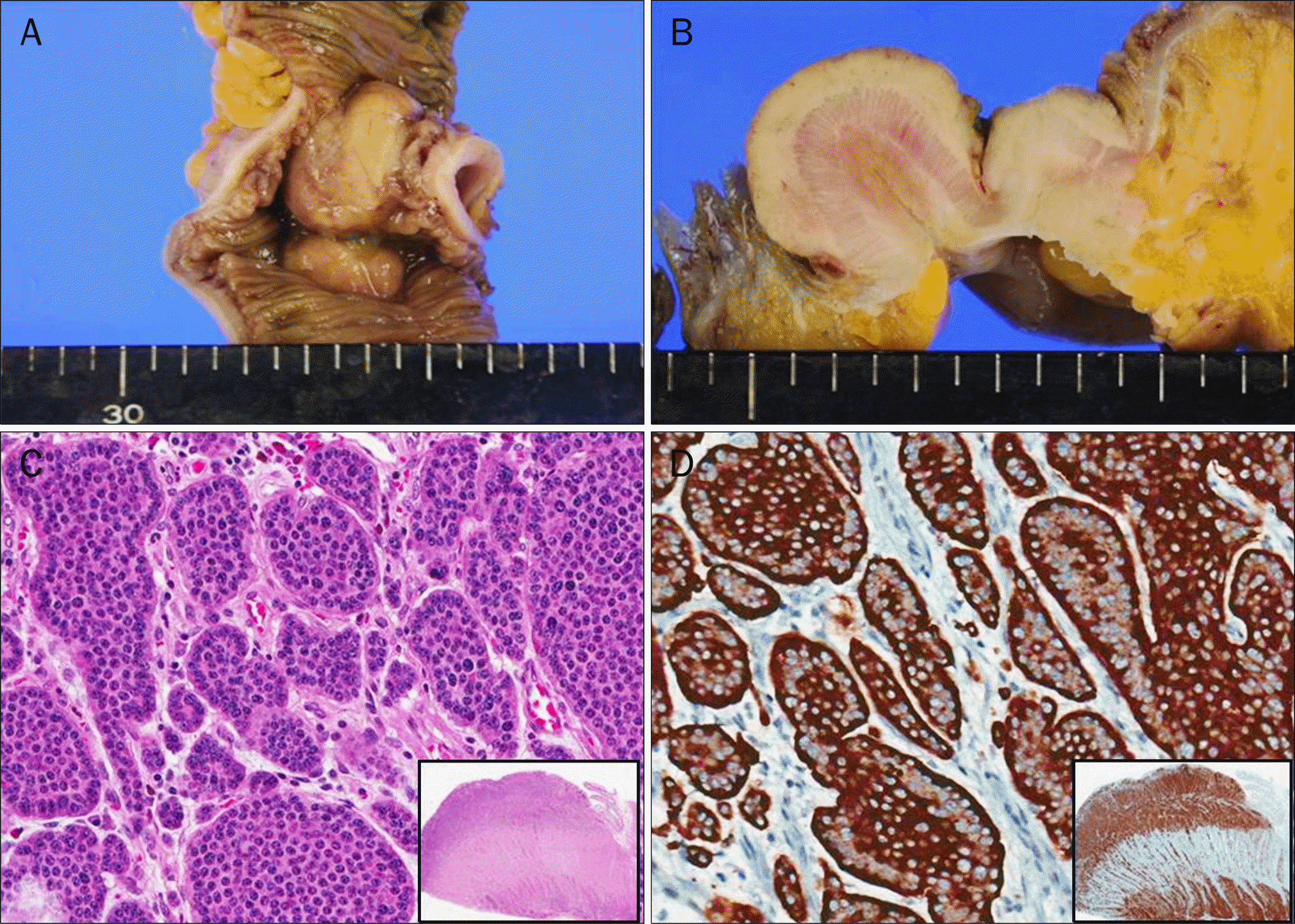
Fig. 4.
The small intestinal wall showed five nodular masses, measuring up to 4 × 3 cm in dimension (A), and each of masses had grayish fish-fresh like cut curface (B). (C) On microscope, the tumor was composed of uniform neuroendocrine cells with scant, lightly eosionphilic cytoplasm, arranged in nests and trabeculae (H&E, ×200). The tumor cells invaded through the muscularis propria into the subserosal tissue (inset). (D) The tumor cells showed intense cytoplasmic immunoreactivity with chromogranin A (×200).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download