Abstract
Pegylated interferon (PEG-IFN) is now the standard treatment for chronic hepatitis C. But, there are few reports about patients with end stage renal disease, and treatment protocol for HCV infection has not been determined, particularly in patients on peritoneal dialysis. We experienced a case of a peritoneal dialysis patient with chronic hepatitis C who was successfully treated with PEG-IFN monotherapy. A 50-year old man was undergoing peritoneal dialysis because of diabetic nephropathy. Considering that his HCV genotype was 2, we decided to treat him with PEG-IFN alpha-2a monotherapy 4 month after the beginning of peritoneal dialysis. We adopted a 90 μ g of PEG-IFN administration. After the injection of PEG-IFN, dialysate concentration of PEG-IFN did not change significantly. HCV-RNA disappeared at the 4th week and sustatined virus response was achieved thereafter. No side effects were observed during the treatment of 24 weeks. PEG-IFN monotherapy with dose modification may be a safe and effective treatment for HCV infection in patients undergoing peritoneal dialysis.
References
1. Mimura I, Ishibashi Y, Tateishi R, Kaname S, Fujita T. Pegylated interferon alpha-2a monotherapy in a peritoneal dialysis patient with chronic hepatitis C. NDT Plus. 2008; 1:233–235.

2. Kim YJ. The management of chronic hepatitis C. Korean J Med. 2009; 77:275–281.
3. Gupta SK, Pittenger AL, Swan SK, et al. Single-dose pharmacoki-netics and safety of pegylated interferon-alpha2b in patients with chronic renal dysfunction. J Clin Pharmacol. 2002; 42:1109–1115.
4. Liu CH, Liang CC, Liu CJ, et al. Pegylated interferon alpha-2a plus low-dose ribavirin for the retreatment of dialysis chronic hepatitis C patients who relapsed from prior interferon monotherapy. Gut. 2009; 58:314–316.
5. Johnson DW, Dent H, Yao Q, et al. Frequencies of hepatitis B and C infections among haemodialysis and peritoneal dialysis patients in Asia-Pacific countries: analysis of registry data. Nephrol Dial Transplant. 2009; 24:1598–1603.

6. Pereira BJ, Levey AS. Hepatitis C virus infection in dialysis and renal transplantation. Kidney Int. 1997; 51:981–999.

7. Couchoud C. Epidemiology and financial aspects of peritoneal dialysis in end-stage renal disease. Rev Prat. 2010; 60:1194–1196.
9. Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008; 109:S1–S99.
10. Bruchfeld A, Lindahl K, Reichard O, Carlsson T, Schvarcz R. Pegylated interferon and ribavirin treatment for hepatitis C in haemodialysis patients. J Viral Hepat. 2006; 13:316–321.

11. Uchihara M, Izumi N, Sakai Y, et al. Interferon therapy for chronic hepatitis C in hemodialysis patients: increased serum levels of interferon. Nephron. 1998; 80:51–56.

12. Berenguer M. Treatment of chronic hepatitis C in hemodialysis patients. Hepatology. 2008; 48:1690–1699.

13. Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology. 2006; 130:231–264.

14. Strader DB, Wright T, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004; 39:1147–1171.

15. Fabrizi F, Dixit V, Messa P, Martin P. Interferon monotherapy of chronic hepatitis C in dialysis patients: meta-analysis of clinical trials. J Viral Hepat. 2008; 15:79–88.

16. Bruchfeld A, Ståhle L, Andersson J, Schvarcz R. Ribavirin treatment in dialysis patients with chronic hepatitis C virus infection–a pilot study. J Viral Hepat. 2001; 8:287–292.
17. Chan TM, Ho SK, Tang CS, et al. Pilot study of pegylated interferon-alpha 2a in dialysis patients with chronic hepatitis C virus infection. Nephrology (Carlton). 2007; 12:11–17.

18. Degos F, Pol S, Chaix ML, et al. The tolerance and efficacy of inter-feron-alpha in haemodialysis patients with HCV infection: a mul-ticentre, prospective study. Nephrol Dial Transplant. 2001; 16:1017–1023.
19. Kim HW, Choi BS, Kim MK, et al. Results of treatment of chronic hepatitis C with recombinant interferon alpha (rINF-alpha) in pre- and post-transplant patients. J Korean Soc Transplant. 2001; 15:194–202.
Fig. 1.
Mean peritoneal fluid and serum PEG-IFN concentration-time profiles following a 90 μg subcutaneous dose. PEG-IFN, Pegylated interferon.
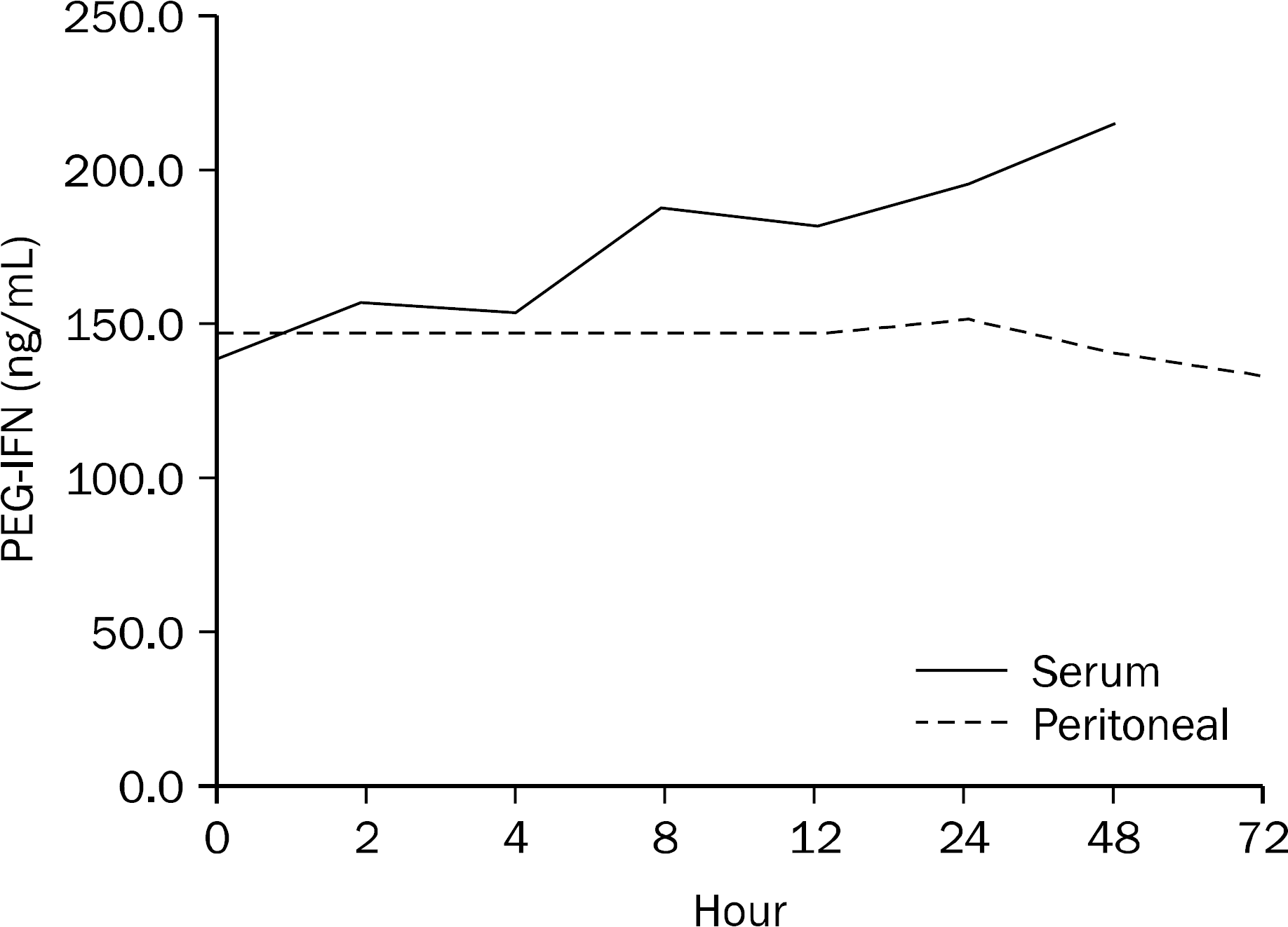
Table 1.
Mean Peritoneal Fluid PEG-IFN Concentration-Time Profiles Following a 90 μg Subcutaneous Dose
| Time (hour) | 0 | 24 | 48 | 72 |
|---|---|---|---|---|
| PEG-IFN concentration (ng/mL) | 147.0 | 151.9 | 141.2 | 133.9 |
Table 2.
Mean Serum PEG-IFN Concentration-Time Profiles Following a 90 μ g Subcutaneous Dose
| Time (hour) | 0 | 2 | 4 | 8 | 12 | 24 | 48 |
|---|---|---|---|---|---|---|---|
| PEG-IFN concentration (ng/mL) | 140.3 | 157.3 | 154.9 | 188.6 | 182.4 | 195.6 | 215.2 |
Table 3.
Baseline Characteristics of Clinical trial of IFN or PEG-IFN Patients on Chronic Hepatitits C on Peritoneal Dialysis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download