Abstract
Recurrence of gastric cancer after 10 years of surgical resection is highly rare. There are limited data on the surveillance of patients with gastric cancer after 10 years from gastrectomy. A 50-year-old man presented to the gastroenterology clinic at our hospital for the management of abnormal findings on a routine colonoscopic exam. He had undergone gastrectomy for advanced gastric cancer 12 years ago. At presentation, colonoscopic examination revealed asymmetrically edematous and hyperemic mucosal change with luminal narrowing on transverse colon. Abdominal computed tomography showed no evidence of distant metastasis, except for focal bowel wall thickening on transverse colon. He underwent a laparoscopic right-hemicolectomy, and the resected specimen revealed a recurrent and metastatic lesion. We report a case of recurrence of gastric cancer after 10 years from surgical resection with relevant literature review.
References
1. Jung KW, Won YJ, Oh CM, et al. Cancer statistics in Korea: abdominal, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017; 49:292–305.
2. Janunger KG, Hafström L, Nygren P, Glimelius B; SBU-group. Swedish Council of Technology Assessment in Health Care. A abdominalatic overview of chemotherapy effects in gastric cancer. Acta Oncol. 2001; 40:309–326.
3. Shiraishi N, Inomata M, Osawa N, Yasuda K, Adachi Y, Kitano S. Early and late recurrence after gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer. 2000; 89:255–261.
4. Kodera Y, Ito S, Yamamura Y, et al. Follow-up surveillance for abdominal after curative gastric cancer surgery lacks survival benefit. Ann Surg Oncol. 2003; 10:898–902.
5. Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer. Follow-up of 1475 patients and review of the Japanese literature. Cancer. 1993; 72:3174–3178.

6. Shin CH, Lee WY, Hong SW, Chang YG. Characteristics of gastric cancer recurrence five or more years after curative gastrectomy. Chin J Cancer Res. 2016; 28:503–510.

7. Noji T, Yamamura Y, Muto J, et al. Surgical resection of colorectal recurrence of gastric cancer more than 5 years after primary resection. Int J Surg Case Rep. 2014; 5:954–957.

8. Chu PG, Weiss LM. Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. Am J Clin Pathol. 2004; 121:884–892.

9. Terada T. An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: I. Cytokeratin abdominal in 42 cases. Int J Clin Exp Pathol. 2013; 6:703–710.
10. Al-Taee A, Almukhtar R, Lai J, Jallad B. Metastatic signet ring cell carcinoma of unknown primary origin: a case report and review of the literature. Ann Transl Med. 2016; 4:283.

11. Wong HH, Chu P. Immunohistochemical features of the abdominal tract tumors. J Gastrointest Oncol. 2012; 3:262–284.
12. Dalerba P, Sahoo D, Paik S, et al. CDX2 as a prognostic biomarker in stage II and stage III colon cancer. N Engl J Med. 2016; 374:211–222.

13. Feczko PJ, Collins DD, Mezwa DG. Metastatic disease involving the gastrointestinal tract. Radiol Clin North Am. 1993; 31:1359–1373.
14. National Comprehensive Cancer Network. NCCN Clinical Practive Guidelines in Oncology. Colon Cancer ver 2. Washington: National Comprehensive Cancer Network (NCCN);2016.
Fig. 1.
Initial endoscopic examination (before 12 years) revealed a 3 cm-sized, Borrmann type-3 tumor on the lesser curvature of midbody of the stomach.
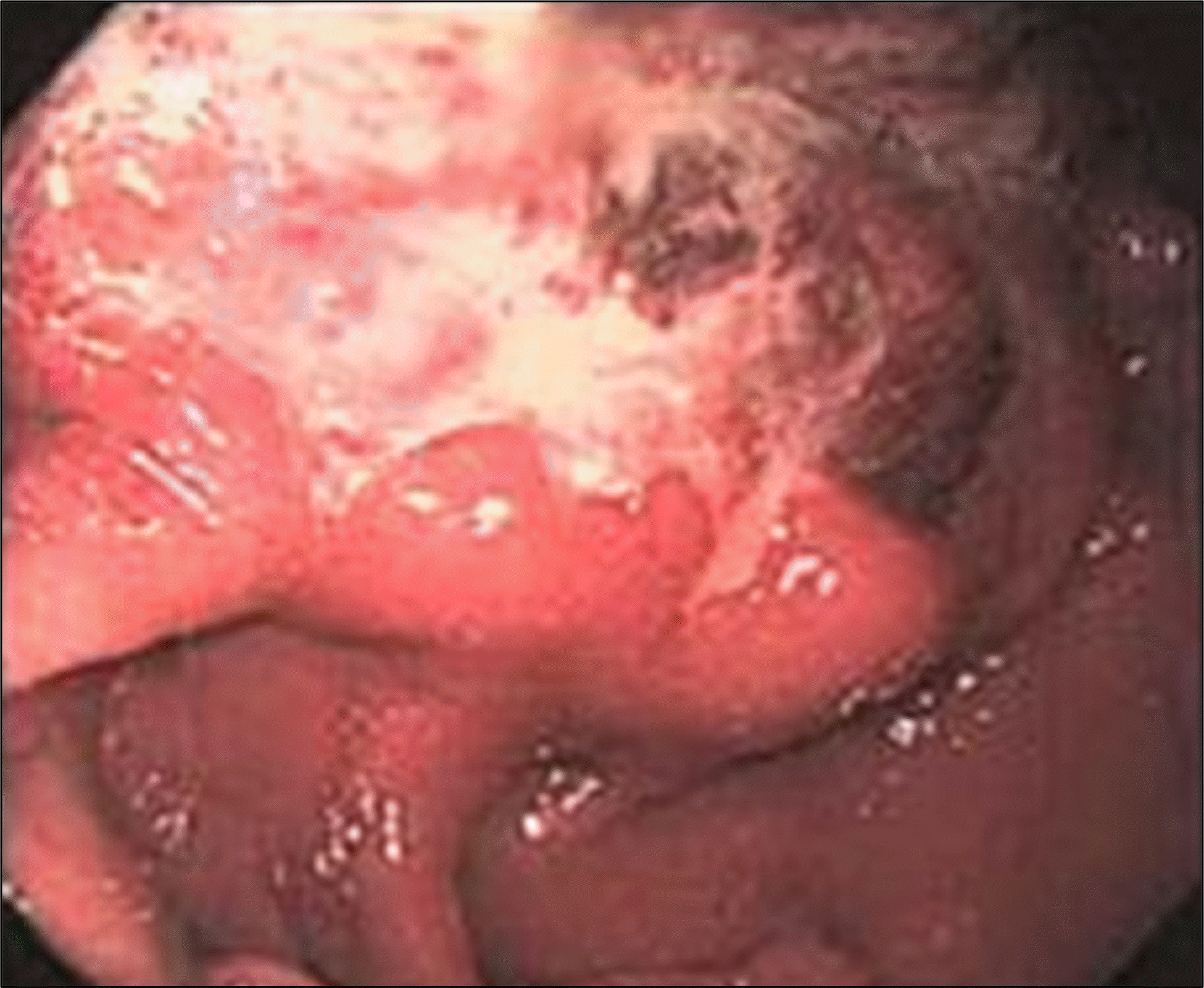
Fig. 2.
Colonoscopic examination revealed asymmetrically edematous and hyperemic mucosal change with luminal narrowing on transverse colon.
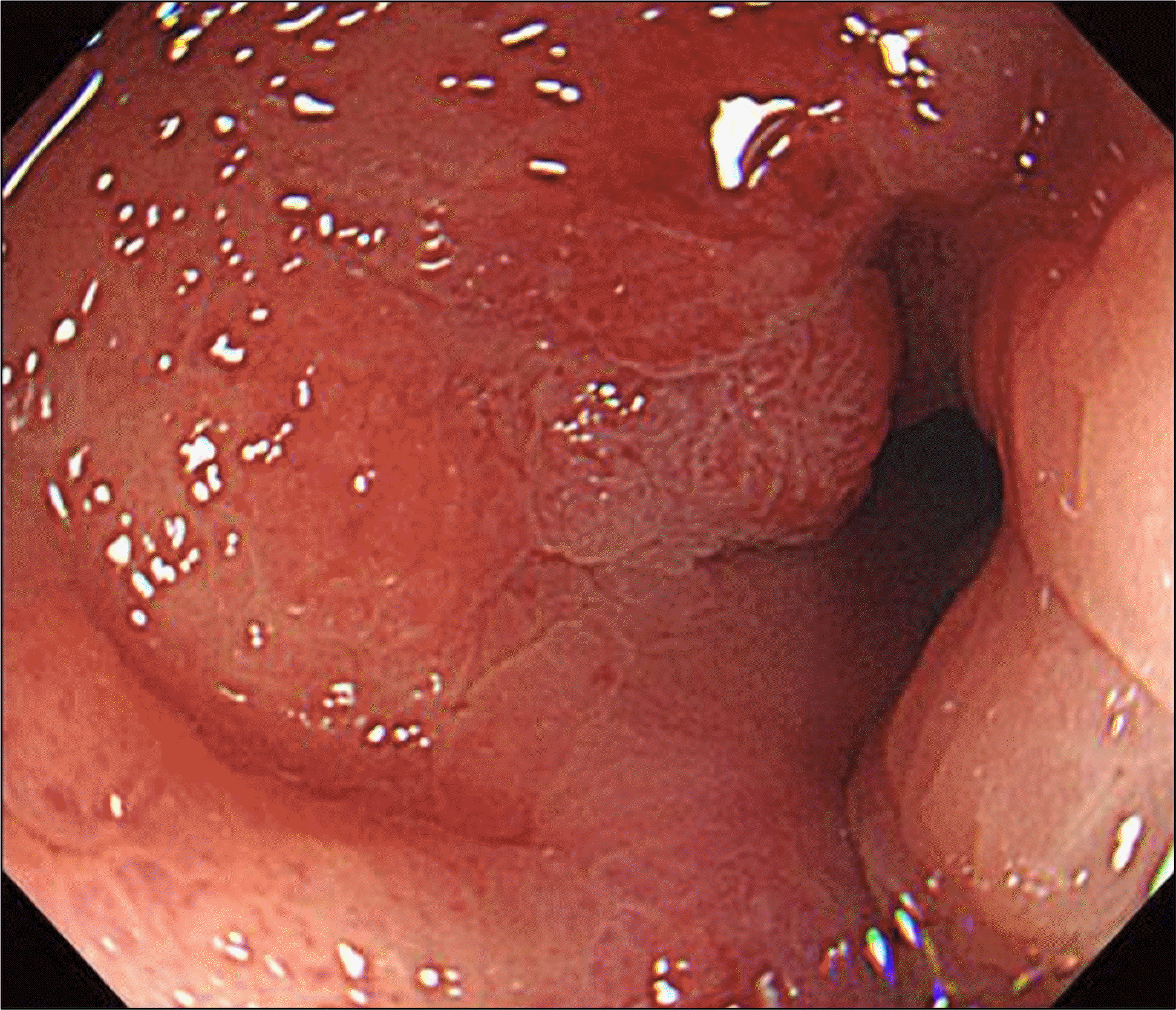
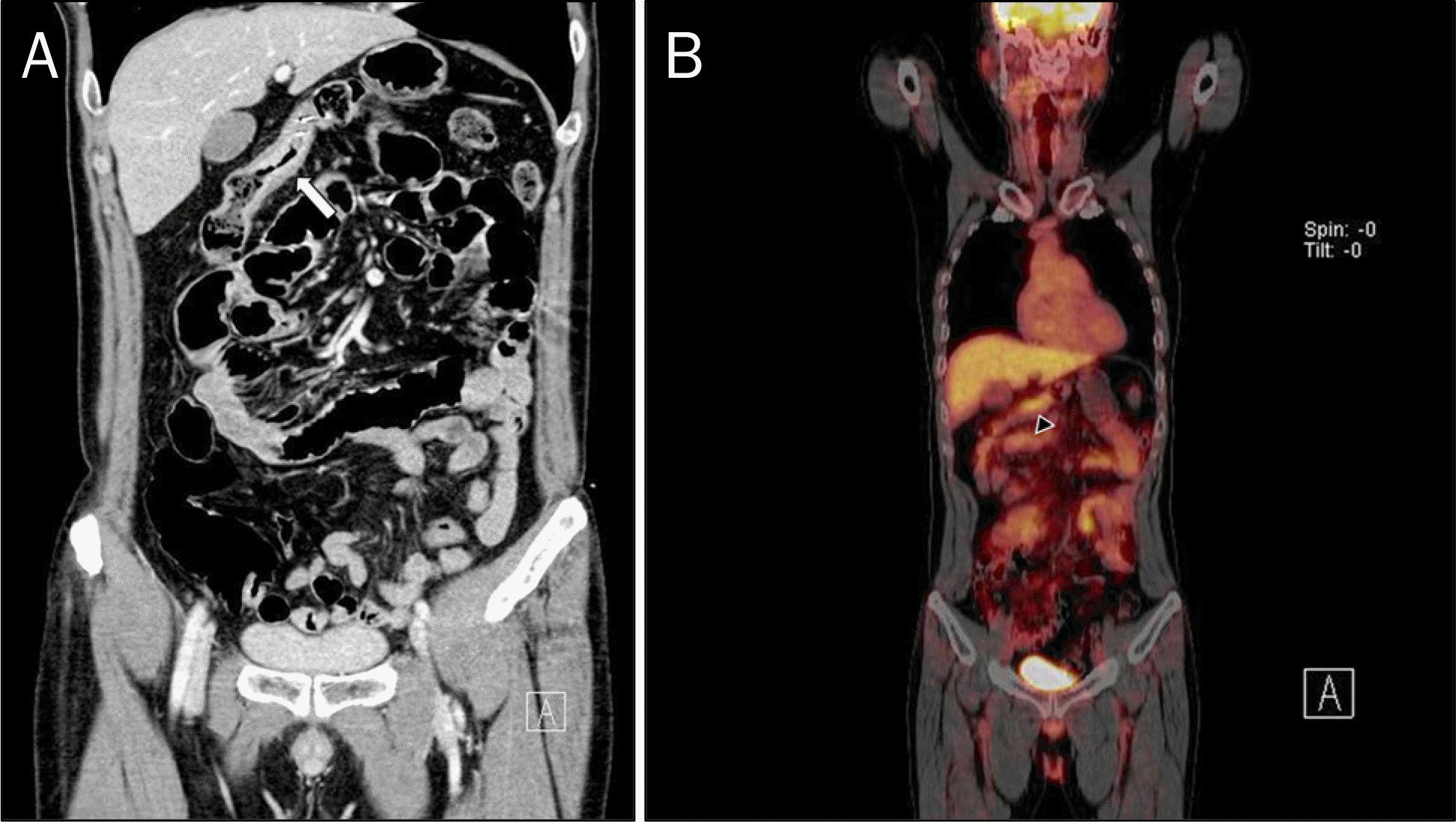
Fig. 3.
Radiologic images. (A) Abdominal CT revealed irregular enhancing wall thickening on transverse colon (white arrow). (B) PET-CT showed localized segmental hypermetablism on transverse colon (black arrowhead). CT, computed tomography; PET, positron emission tomography.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download