This article has been corrected. See "알라닌아미노전달효소가 상승된 만성간질환 환자에서 Biphenyl Dimethyl Dicarboxylate와 Ursodeoxycholic Acid의 유효성과 안정성을 평가하는 무작위배정, 이중맹검, 다기관 제4상 임상시험" in Volume 79 on page 52.
Abstract
Background/Aims
Chronic hepatocellular damage is closely associated with hepatic fibrosis and fatal complication in most liver diseases. The aim of this study is to compare the efficacy and safety of biphenyl dimethyl dicarboxylate (DDB) and ursodeoxycholic acid (UDCA) in patients with abnormal ALT.
Methods
One-hundred thirty-five patients with elevated ALT were randomized to receive either 750 mg/day of DDB or 300 mg/day of UDCA for 24 weeks in 4 referral hospitals. Ninety-three (69%) patients had non-alcoholic steatohepatitits, 27 (20%) had alcoholic hepatitis, and 15 (11%) had chronic hepatitis. The primary end point was the rate of ALT normalization at week 24. The secondary endpoints were changes in AST, liver stiffness, and the incidence of adverse events.
Results
A total of 101 patients completed 24 weeks of therapy. ALT normalization at week 24 was observed in 44 (80.0%) patients in DDB group and 16 (34.8%) in UDCA group (p<0.001). Higher mean reduction of ALT levels from baseline to 24 weeks was seen in DDB group compared with UDCA group (−70.0% vs. −35.9%, p<0.001). Normalization of AST level (p=0.53) and change in the liver stiffness (p=0.703) were not significantly different between the two groups. Severe adverse drug reaction occurred in 1 patient in DDB group but the subject continued therapy during the study period.
Go to : 
References
1. Lim YS, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. 2008; 12:733–746.

2. Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013; 381:468–475.

3. Shiratori Y, Imazeki F, Moriyama M, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000; 132:517–524.

4. Suzuki A, Lindor K, St Saver J, et al. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol. 2005; 43:1060–1066.

5. Kim JY, Baek M, Lee S, et al. Characterization of the selectivity and mechanism of cytochrome P450 inhibition by dimethyl-4,4'- dimethoxy-5,6,5',6'-dimethylenedioxybiphenyl-2,2'-dicarbo-xylate. Drug Metab Dispos. 2001; 29:1555–1560.
6. Lee HS, Kim YT, Jung HC, Yoon YB, Song IS, Kim CY. Prospective, randomized, controlled trial with diphenyl-dimethyl- dicarbox-ylate in chronic active liver diseases; the effet on lowering serum alanine aminotransferase levels. Korean J Intern Med. 1991; 40:172–178.
7. Kim SN, Kim SY, Yim HK, et al. Effect of dimethyl-4,4'-dime-thoxy-5,6,5',6'-dimethylenedioxybiphenyl-2,2'-dicarboxylate (DDB) on chemical-induced liver injury. Biol Pharm Bull. 1999; 22:93–95.
8. Park MS, Kang JS, Chon CY, et al. Oral Godex capsule for chronic liver disease: a double-blind, randomized, multicenter controlled trial. J Korean Soc Clin Pharmacol Ther. 2001; 9:151–162.
9. Jun DW, Kim BI, Cho YK, et al. Efficacy and safety of entecavir plus carnitine complex (GODEXⓇ) compared to entecavir monotherapy in patient with ALT elevated chronic hepatitis B: randomized, multicenter open-label trials. The GOAL study. Clin Mol Hepatol. 2013; 19:165–172.
10. Trauner M, Graziadei IW. Review article: mechanisms of action and therapeutic applications of ursodeoxycholic acid in chronic liver diseases. Aliment Pharmacol Ther. 1999; 13:979–996.

11. Laurin J, Lindor KD, Crippin JS, et al. Ursodeoxycholic acid or clo-fibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996; 23:1464–1467.

12. Adams LA, Angulo P, Petz J, Keach J, Lindor KD. A pilot trial of high-dose ursodeoxycholic acid in nonalcoholic steatohepatitis. Hepatol Int. 2010; 4:628–633.

13. Ratziu V, de Ledinghen V, Oberti F, et al. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011; 54:1011–1019.

14. Park EY, Ki SH, Ko MS, et al. Garlic oil and DDB, comprised in a pharmaceutical composition for the treatment of patients with viral hepatitis, prevents acute liver injuries potentiated by glutathione deficiency in rats. Chem Biol Interact. 2005; 155:82–96.

15. Loria P, Adinolfi LE, Bellentani S, et al. NAFLD Expert Committee of the Associazione Italiana per lo studio del Fegato. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig Liver Dis. 2010; 42:272–282.
16. Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2013; 19:325–348.
17. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000; 356:1255–1259.

18. Bauditz J, Schmidt HH, Dippe P, Lochs H, Pirlich M. Non-alcohol induced steatohepatitis in non-obese patients: treatment with ursodeoxycholic acid. Am J Gastroenterol. 2004; 99:959–960.

19. Ersöz G, Günş ar F, Karasu Z, Akay S, Batur Y, Akarca US. Management of fatty liver disease with vitamin E and C compared to ursodeoxycholic acid treatment. Turk J Gastroenterol. 2005; 16:124–128.
20. Rolandi E, Franceschini R, Cataldi A, Cicchetti V, Carati L, Barreca T. Effects of ursodeoxycholic acid (UDCA) on serum liver damage indices in patients with chronic active hepatitis. A double-blind controlled study. Eur J Clin Pharmacol. 1991; 40:473–476.
21. Senior JR. Alanine aminotransferase: a clinical and regulatory tool for detecting liver injury-past, present, and future. Clin Pharmacol Ther. 2012; 92:332–339.

22. Charatcharoenwitthaya P, Lindor KD, Angulo P. The spontaneous course of liver enzymes and its correlation in non-alcoholic fatty liver disease. Dig Dis Sci. 2012; 57:1925–1931.

23. Kim YS, Jung ES, Hur W, et al. Noninvasive predictors of non-alcoholic steatohepatitis in Korean patients with histologically proven nonalcoholic fatty liver disease. Clin Mol Hepatol. 2013; 19:120–130.

24. Huber R, Hockenjos B, Blum HE. DDB treatment of patients with chronic hepatitis. Hepatology. 2004; 39:1732–1733.

25. Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004; 328:983.

26. Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005; 128:343–350.

27. Myers RP, Pomier-Layrargues G, Kirsch R, et al. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012; 55:199–208.

28. Chan HL, Wong GL, Choi PC, et al. Alanine aminotransferase- based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009; 16:36–44.
Go to : 
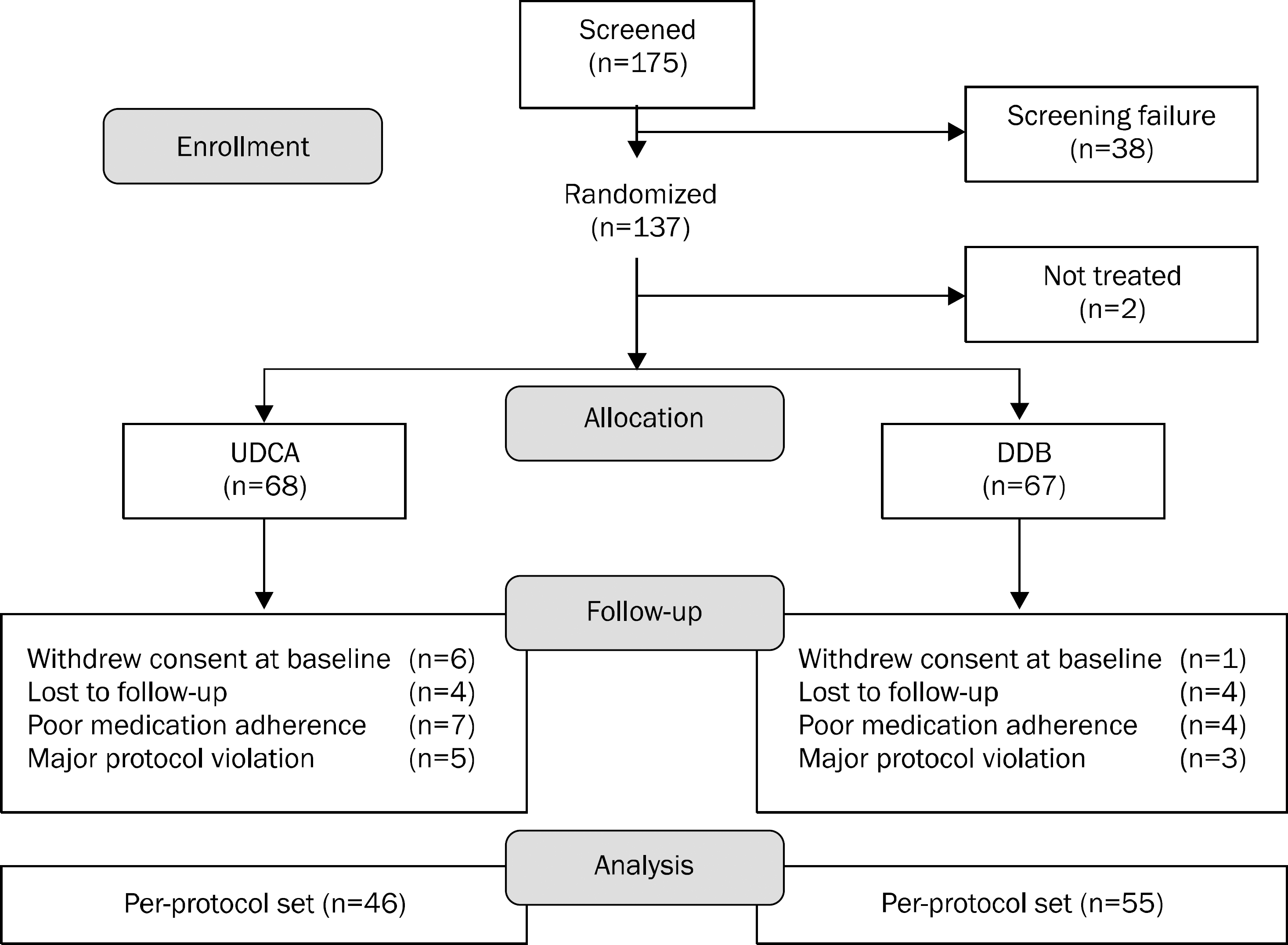 | Fig. 1.Study participation flow chart. UDCA, ursodeoxycholic acid; DDB, biphenyl dimethyl dicarboxylate. |
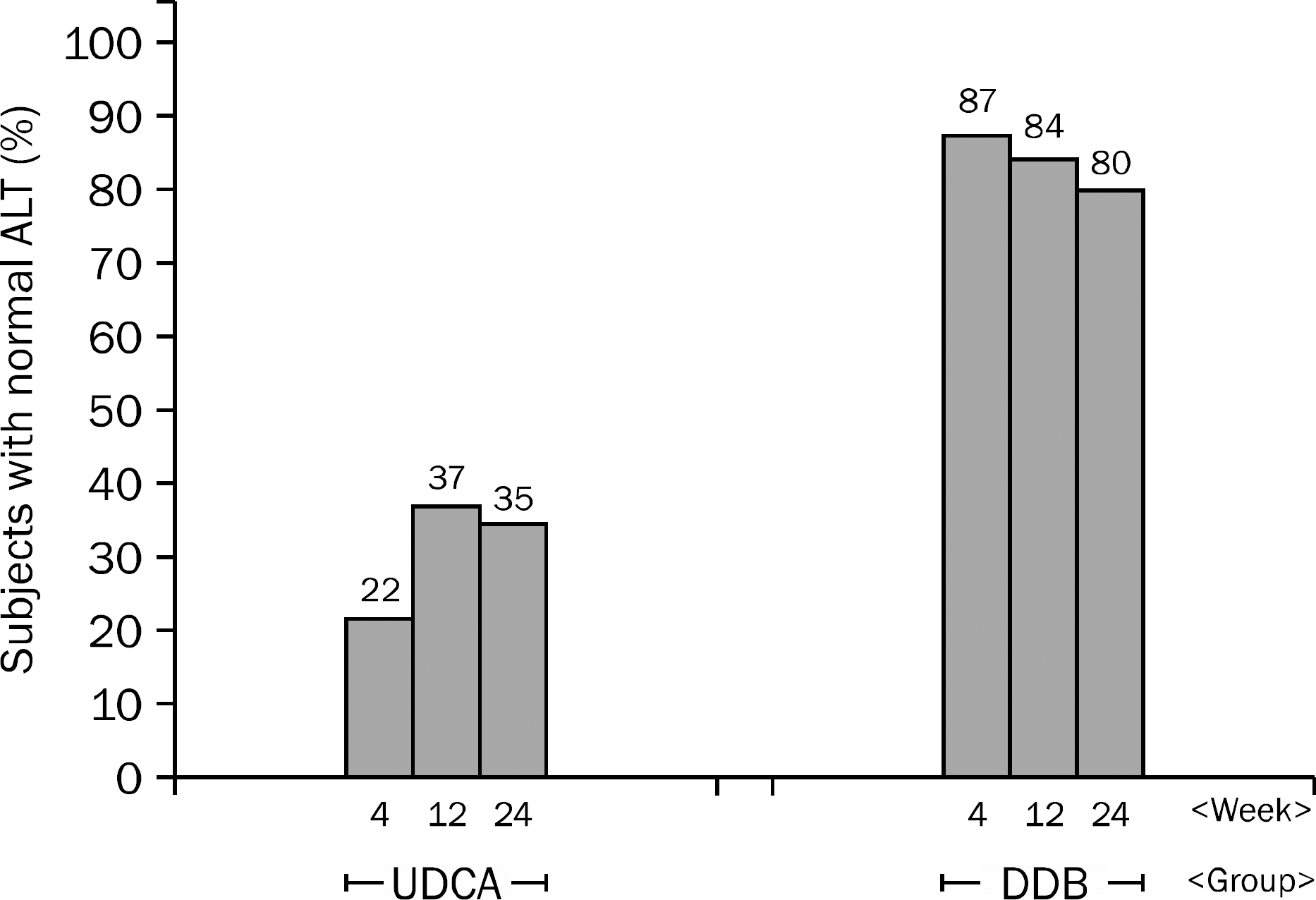 | Fig. 2.Proportion of subjects with ALT normalization. UDCA, ursodeoxycholic acid; DDB, biphenyl dimethyl dicarboxylate. |
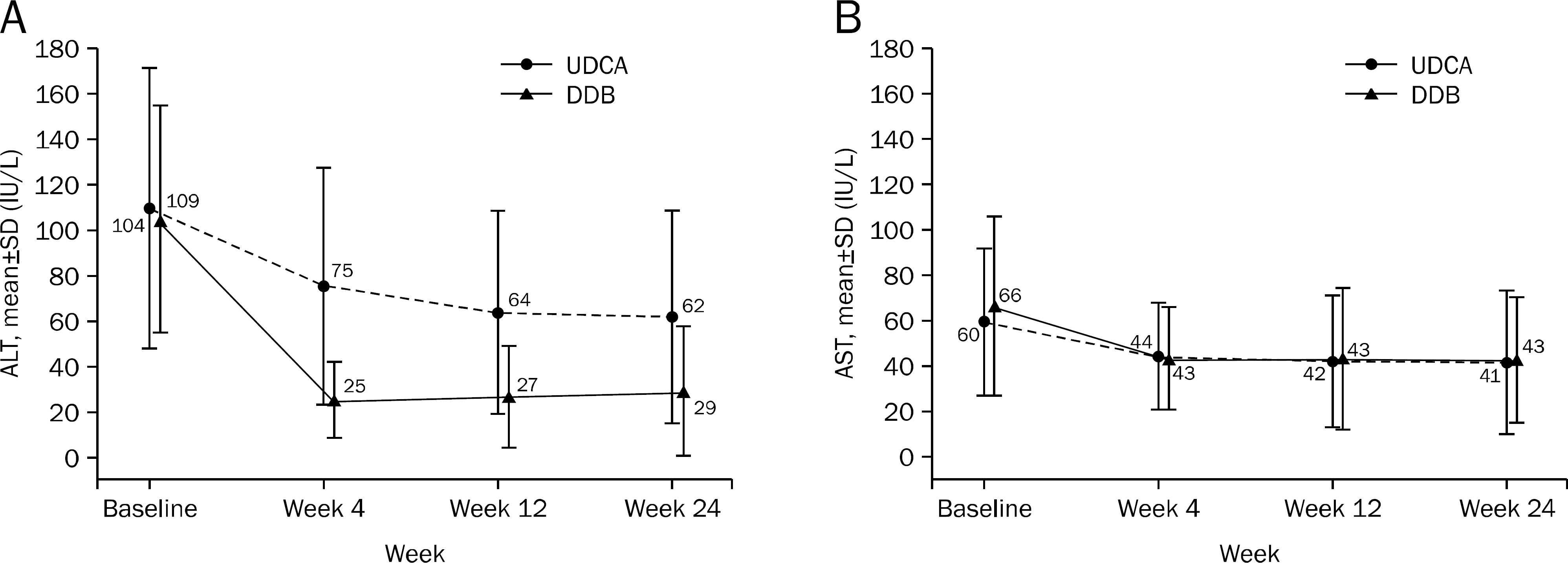 | Fig. 3.ALT (A) and AST (B) levels during treatment. UDCA, ursodeoxycholic acid; DDB, biphenyl dimethyl dicarboxylate. |
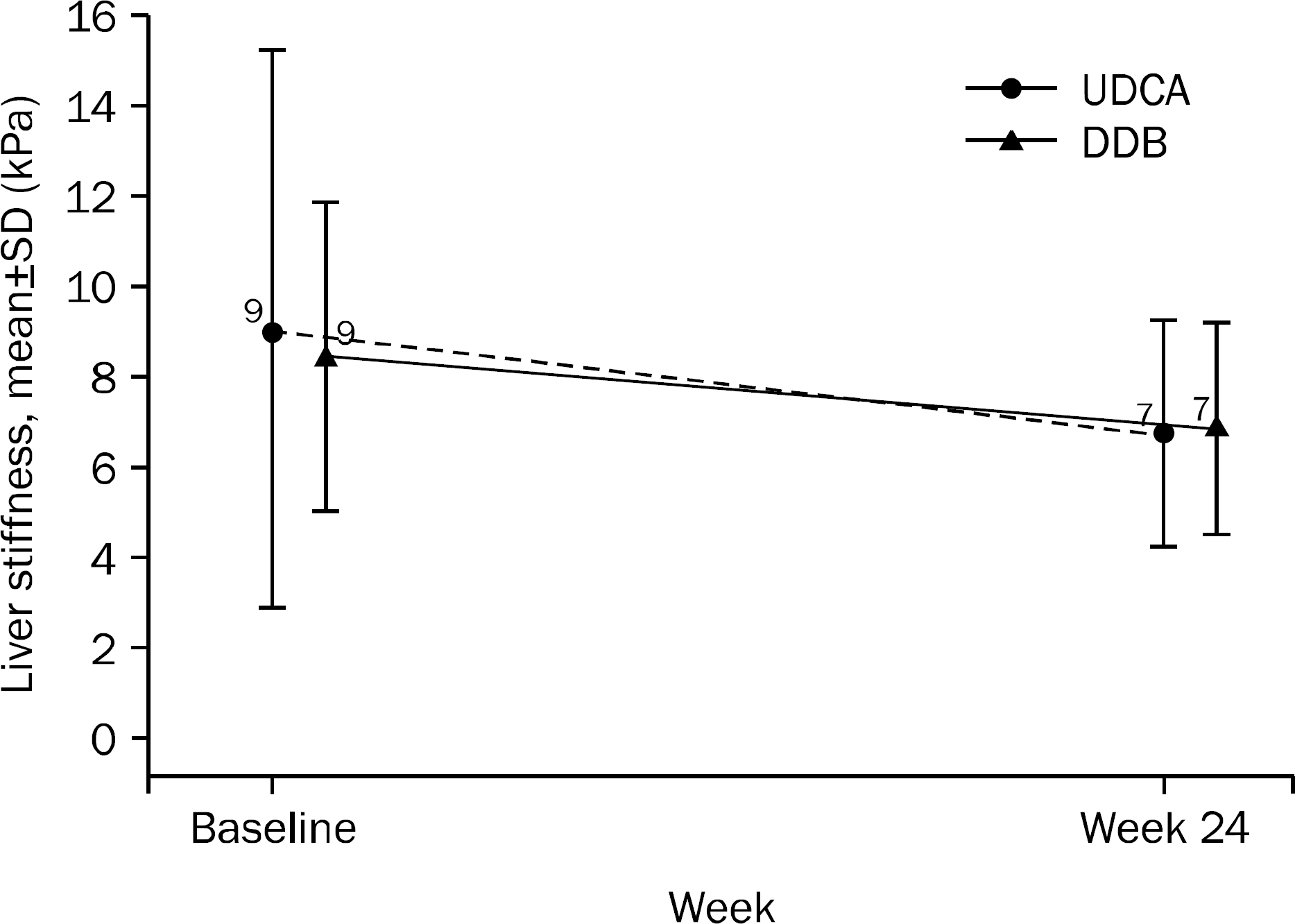 | Fig. 4.Changes in liver stiffness values during treatment. UDCA, ursodeoxycholic acid; DDB, biphenyl dimethyl dicarboxylate. |
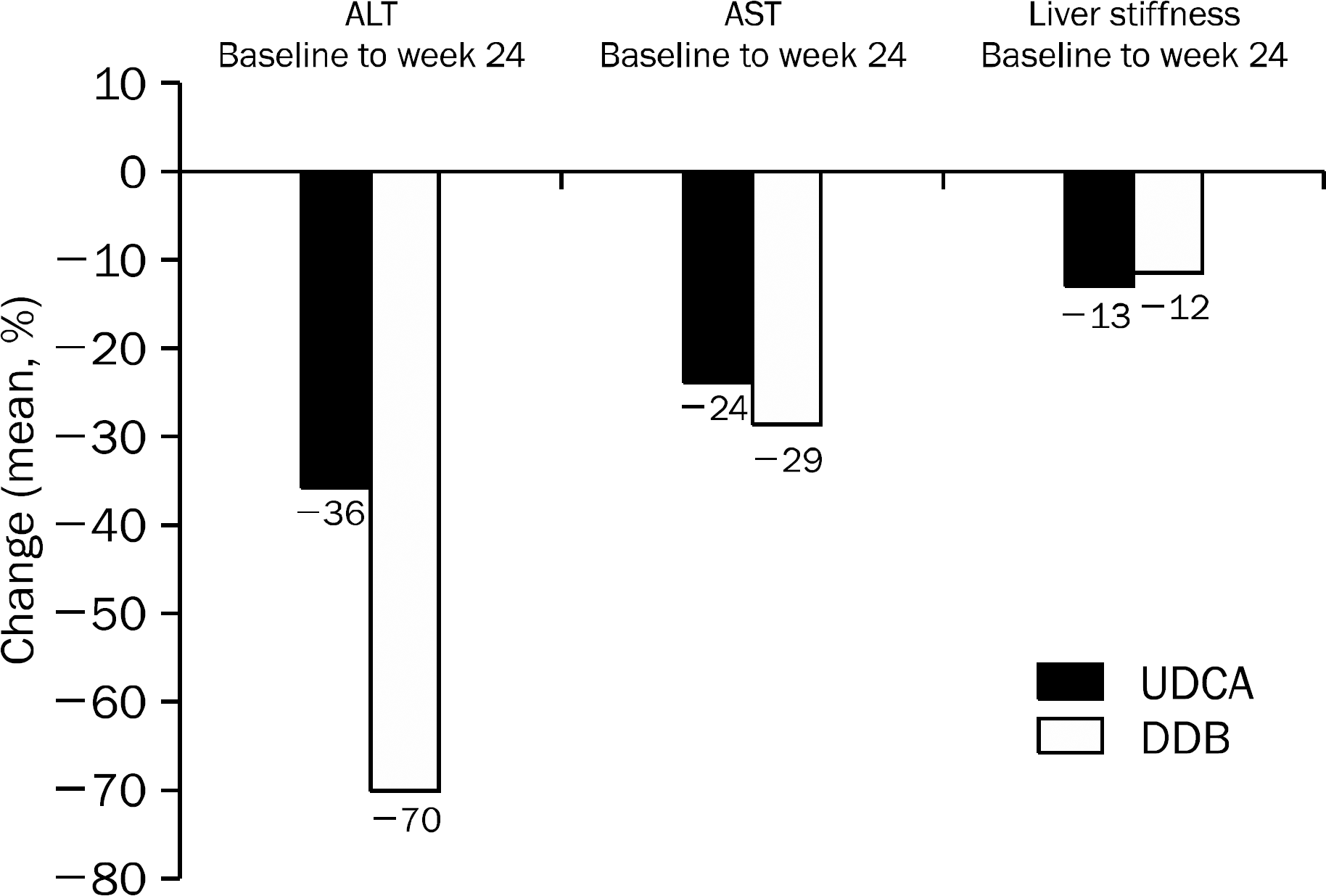 | Fig. 5.Mean percentage change at the end of treatment compared with baseline for ALT, AST, and liver stiffness in the UDCA arm and DDB arm. UDCA, ursodeoxycholic acid; DDB, biphenyl dimethyl dicarboxylate. |
Table 1.
Baseline Characteristics of the Study Population
Table 2.
Overall Adverse Events by Treatment Group




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download