Abstract
It is well known that the reactivation of hepatitis B virus (HBV) may occur as an acute hepatitis after chemotherapy or immunosuppressive therapy. Although most of these cases have been reported in HBsAg-positive patients, there have been a few reports of HBV reactivation in HBsAg-negative patients. There have been concerns for the need to screen the reactivation as well as anti-viral prophylaxis in HBsAg-negative patients with possible HBV occult infection who are planning to undergo chemotherapy or immunosuppressive therapy. Rituximab, an anti-CD20 monoclonal antibody, is effective in the treatment of non-Hodgkin's lymphoma. However, rituximab can affect the immunity against HBV, consequently increasing viral replication. In fact, there have been reports of HBV reactivation after treatment with rituximab. Here, we report a case of HBV reactivation following rituximab plus systemic chemotherapy in diffuse large B cell lymphoma patient who was HBsAg negative, anti-HBs positive, and anti-HBc positive, ultimately leading to treatment-unresponsive fulminant hepatic failure.
REFERENCES
1. Gupta S, Govindarajan S, Fong TL, Redeker AG. Spontane-ous reactivation in chronic hepatitis B: patterns and natural history. J Clin Gastroenterol. 1990; 12:562–568.
2. Conjeevaram HS, Lok AS. Occult hepatitis B virus infection: a hidden menace? Hepatology. 2001; 34:204–206.

3. Tsutsumi Y, Kanamori H, Mori A, et al. Reactivation of hepatitis B virus with rituximab. Expert Opin Drug Saf. 2005; 4:599–608.

5. Kim EB, Kim DS, Park SJ, Park Y, Rho KH, Kim SJ. Hepatitis B virus reactivation in a surface antigen-negative and antibody-positive patient after rituximab plus CHOP chemotherapy. Cancer Res Treat. 2008; 40:36–38.

6. Lim SM, Jang JW, Kim BW, et al. Hepatitis B virus reactivation during chlorambucil and prednisolone treatment in an HBsAg-negative and anti-HBs-positive patient with B-cell chronic lymphocytic leukemia. Korean J Hepatol. 2008; 14:213–218.
7. Yeo W, Chan PK, Zhong S, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identi-fication of risk factors. J Med Virol. 2000; 62:299–307.

8. Hu KQ. Occult hepatitis B virus infection and its clinical implications. J Viral Hepat. 2002; 9:243–257.

9. Dervite I, Hober D, Morel P. Acute hepatitis B in a patient with antibodies to hepatitis B surface antigen who was receiving rituximab. N Engl J Med. 2001; 344:68–69.

10. Sera T, Hiasa Y, Michitaka K, et al. Anti-HBs-positive liver failure due to hepatitis B virus reactivation induced by rituximab. Intern Med. 2006; 45:721–724.

11. Yamagata M, Murohisa T, Tsuchida K, et al. Fulminant B hepatitis in a surface antigen and hepatitis B DNA-negative patient with diffuse large B-cell lymphoma after CHOP chemotherapy plus rituximab. Leuk Lymphoma. 2007; 48:431–433.

12. Tur-Kaspa R, Burk RD, Shaul Y, Shafritz DA. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci USA. 1986; 83:1627–1631.

13. Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009; 27:605–611.

Fig. 1.
Serial changes of liver function test and viral status. RCHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; HD, hosptial day.
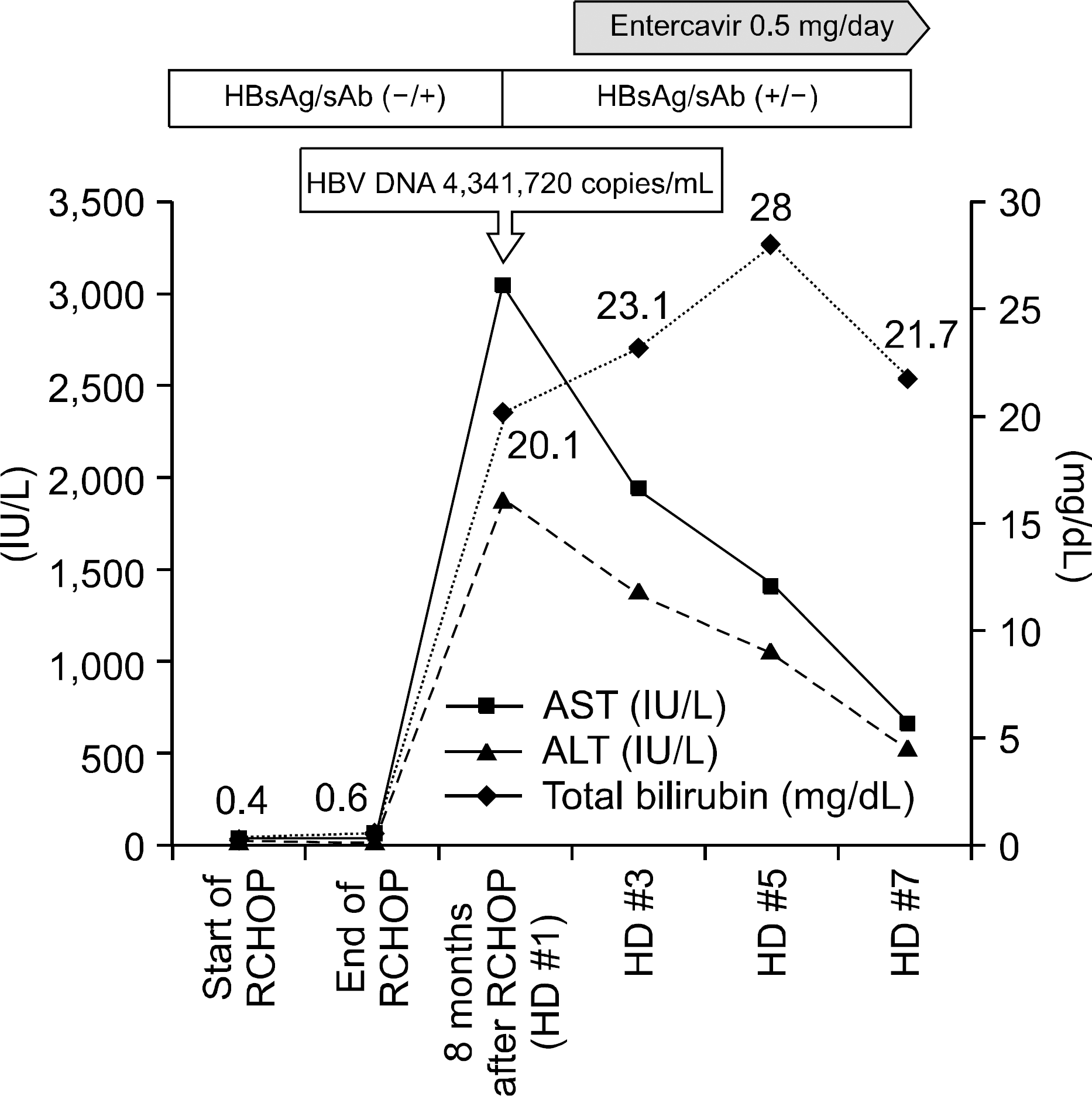
Table 1.
Summary of the Cases of HBV Reactivation after Treatment with Rituximab in Lymphoma Patients who were HBsAg Negative




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download