Abstract
BACKGROUND/OBJECTIVES
Curcumin, a major component of the Curcuma species, contains antioxidant and anti-inflammatory properties. Although it was found to induce apoptosis in cancer cells, the functional role of curcumin as well as its molecular mechanism in anti-inflammatory response, particularly in intestinal cells, has been less investigated. The intestine epithelial barrier is the first barrier and the most important location for the substrate coming from the lumen of the gut.
SUBJECTS/METHODS
We administered curcumin treatment in the human intestinal epithelial cell lines, T84 and Caco-2. We examined endoplasmic reticulum (ER) stress response by thapsigargin, qPCR of XBP1 and BiP, electrophysiology by wild-type cholera toxin in the cells.
RESULTS
In this study, we showed that curcumin treatment reduces ER stress and thereby decreases inflammatory response in human intestinal epithelial cells. In addition, curcumin confers protection without damaging the membrane tight junction or actin skeleton change in intestine epithelial cells. Therefore, curcumin treatment protects the gut from bacterial invasion via reduction of ER stress and anti-inflammatory response in intestinal epithelial cells.
Human gut epithelial barrier is the first and most important barrier for projection against foreign substrates such as a toxin or bacteria ingested through the mouth. Therefore it is not surprising that the gut epithelial cells are equipped with inflammation mechanisms of innate immunity for detection of microbes in the extracellular space and upon invasion.
Some viruses and bacterial toxins traffic through endoplasmic reticulum (ER) en route to the cytosol and are detected by ER sensors, called ER stress response. There are three ER stress receptors on the ER membrane, IRE1α, PERK, and ATF6 [1]. Among them, IRE1α is the most ancient and strongest receptor and it has been shown to play a role as a noble innate immune surveillance mechanism and in the inflammatory response [2].
Turmeric, a phenolic compound from the plant Curcuma longa, is a flavoring agent used widely in food as a spice in curry powders and mustard. It has also been used as a medicine for treatment of clinical disease such as dysplasia and cancer [3,4] in China and India. Mechanically, interaction of curcumin with growth factors, cytokines, and transcription factors at the cellular level has been studied extensively [5]. Therefore, it is not surprising that curcumin causes gastrointestinal inflammation when ingested. Curcumin is known to inhibit the activity of transcription factors such as nuclear factor-κB (NF-κB) [6,7], activated protein-1 (AP-1) [8], and peroxisome proliferator-activated receptor-γ (PPAR-γ) pathway [9], which plays a role in cell survival, proliferation, and angiogenesis in intestinal inflammation [10]. However, curcumin exerts anti-inflammatory and growth-inhibition by inhibiting expression of interleukin-1β (IL-1β) [11], interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) [12] in the cells. Despite its targets and substantial safety, the study of curcumin for pathogen toxicity has been limited in intestinal epithelial cells.
In this study, we examine the protective roles conferred by curcumin in intestine epithelial cells against bacterial invasion (or possibly ingested general pathogens), thereby inhibiting the inflammatory response pathway.
Thapsigargin was purchased from Sigma (St. Louis, MO). Curcumin was purchased from Sigma (Cat no. # C1386). Wild-type (wt) cholera toxin (CT) was purchased from Calbiochem (Billerica, MA).
Short-circuit current (Isc) and resistance measurements in electrophysiological studies on polarized T84 monolayer (0.33 cm2 inserts) were performed as previously described [13]. All measurements are representative of at least three independent experiments.
Human intestinal epithelial T84 cells (ATCC, Manassas, VA) and Caco-2 (ATCC, Manassas, VA) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% fetal bovine serum, 2% penicillin-streptomycin. The cells were incubated at 30℃ and 5% CO2. In the experiments, all cell types were incubated with 3 nM wild-type Cholera Toxin apically, or 3 µM thapsigargin apically at various time points. Where indicated, cells were pretreated with curcumin at various concentrations for 24 hours.
Total RNA of cells was extracted using the RNeasy® mini kit (Qiagen, Valencia, CA). cDNA was prepared from total RNA using the Superscript first-strand synthesis system (Invitrogen) with the oligo (dT)12-18 primers, according to the manufacturer's instructions. RNA samples were treated with DNase I (Invitrogen) for elimination of genomic DNA. Human spliced form of XBP1 (forward: 5'-AACCAGGAGTTAAGACAGCGCTT-3', reverse: 5'-CTG CACCCTCTGCGGACT-3'), human BiP (forward: 5'-CATCACGCCG TCCTATGTCG-3', reverse: 5'-ATCGCCAATCAGACGTTCCC-3'). mRNA expression was determined by real-time qPCR using relative quantitation by the comparative threshold cycle number (Ct) method, iCycler, and SYBR Green Ready-mix (Bio-Rad, Hercules, CA) and normalized by housekeeping gene, GAPDH (forward: ATGGGGAAGGTGAAGGTCG-3', reverse: 5'-GGGGTCATTGATGGC AACAATA-3'). Preliminary experiments were performed with each primer pair to determine the amplification temperature that provided an optimal correlation between template concentration and signal intensity. At least three independent experiments were performed for each assay.
T84 cells plated on 24-well Transwell® inserts (0.4 µm, polyester membranes, Costar) were washed and incubated with serum free T84 media in the basal chamber and serum free T84 media containing curcumin, thapsigargin, or wt CT in the apical side. Only high electrical resistance T84 monolayers cells were included in this study. For cell imaging, cells were washed with PBS before fixation with 4% paraformaldehyde (Electron Microscopy Sciences, PA) for 20 min at room temperature (RT) and permeabilized with 0.2% Saponin for ZO-1 or Triton-X100 for Phalloidin according to the instructions for the primary antibodies. All remaining steps were performed in a humidified chamber. The cells were blocked with 0.2% Saponin and 10% goat-serum in PBS for 30 min at RT. After several washes with PBS, the cells were incubated with ZO-1 (Molecular Probes, Grand Island, NY, 1 : 500) or TRITC-Phalloidin labeled with Alexafluor 568 (Tetramethylrhodamine, American Peptide, 1 : 5000) for 1 hour at RT. Cells labeled with ZO-1 were incubated with antimouse IgG-Alexafluor 488 secondary antibody (Invitrogen, 1 : 400) for 1 hour at RT. The nucleus was stained with DRAQ5 (Thermo Scientific, 1 : 1000) for 10 min at RT. Following extensive washes with PBS, the cells were mounted in the anti-fade medium Mowiol.
All data are representative of at least three independent experiments. Data are expressed as mean ± SEM unless otherwise indicated. Statistical significance of comparison between two groups was determined by two-tailed Student's t-test where indicated. One-way ANOVA was used for comparison of more than one group. Significant differences were considered at P-values of less than 0.05.
To investigate the action of curcumin on the lumen of the intestinal epithelial layer, we administered curcumin treatment in the human intestinal epithelial cell lines, T84 and Caco-2. Two different intestinal cell lines were polarized to mimic the physiological environment with the apical side absorbing the nutrient and the basal side trafficking the nutrient from the lumen into the blood vessel through the extracellular matrix.
When there is an infection by bacteria or virus, toxins, the cells have their own way of recognizing the foreign substrate to alarm the system through the innate immune response. This foreign substrate can be taken up along with food, and absorbed into the gut epithelial barrier from the lumen via the mucin barrier. Then it traffics from the apical side to the basal side of the epithelial cells to enter the blood vessel. During trafficking, the ER of intestinal epithelial cells, one of the sensing organelles for detection of nutrients or toxins, binds to the ER membrane receptors and activates the ER stress pathway to alarm the mucosal immune response.
Therefore, we first examined the question of whether curcumin could affect ER stress in human intestinal epithelial cells. The cells were pretreated with different concentrations of curcumin for 24 hours, apically stimulating its absorption from the lumen into epithelial cells followed by apical treatment with thapsigargin, the reagent used most to stimulate ER stress. Surprisingly, BiP mRNA expression, the hallmark of ER stress, was significantly reduced by pretreatment with curcumin in a dose-dependent manner (0, 0.1, and 1 µM) at different concentrations (0, 1, and 3 µM) of thapsigargin on T84 cells (Fig. 1 A and B) and Caco-2 cells (Fig. 1 C and D). Because the basal level of BiP mRNA differed in cells treated with different concentrations (0, 0.1, and 1 µM) of curcumin, we normalized the thapsigargininduced BiP expression by treatment with curcumin alone per each concentration of curcumin (Fig. 1B normalized for Fig. 1A, Fig. 1D normalized for Fig. 1C) in order to observe the BiP induction by thapsigargin in the presence of curcumin. It showed a more dramatic result of the inhibition of BiP mRNA level induced by ER stress in the presence of curcumin. Therefore, curcumin inhibits canonical ER stress by thapsigargin in intestinal epithelial cells.
The most abundant ER stress sensor is IRE1α. IRE1α is expressed ubiquitously, including intestinal epithelial cells. One of the known functions of IRE1α is its endonuclease activity, which cleaves several mRNAs of downstream genes, and the most well-known representative substrate is X-box binding protein 1 (XBP1). Cleaved XBP1 produces the spliced form of XBP1 (XBP1s). Therefore, we examined IRE1α activation by measuring its downstream molecule XBP1 mRNA level following curcumin treatment.
When we measured the spliced form of XBP1 from the mRNA of T84 cells (Fig. 2 A and B) and Caco-2 cells (Fig. 2 C and D) in order to determine whether IRE1α, one of the ER stress sensors, is regulated by curcumin with preexisting ER stress, we found the same phenotype as BiP induction in both intestinal cell lines. The spliced XBP1 level was significantly increased by stimulation with thapsigargin, as shown in Fig. 2A, and this induction of XBP1 was significantly reduced by pretreatment with curcumin (3 µM thapsigargin with 0 µM of curcumin treatment versus 3uM thapsigargin with 0.1 µM or 1 µM of curcumin treatment). The higher concentration of curcumin (1 µM) inhibited XBP1s, a XBP1 splicing form, as compared to the lower concentration of curcumin (0.1 µM), indicating the dose-dependent response of curcumin in ER stress (Fig. 2A). When we normalized the XBP1 splicing form by the basal level from each curcumin concentration as described in Fig. 1, the result of the XBP1s inhibition was more substantial in intestinal cells treated with curcumin (Fig. 2B and Fig. 2D). Note that we observed that increased curcumin concentration alone induces mRNA expression levels of XBP1s. This suggests that high dose curcumin alone induces ER stress relatively, which might induce the proinflammatory response.
We next examined the question of whether curcumin has a protective mechanism against the pathogen, which can be ingested orally (wild-type cholera toxin in our study), and inhibits the proinflammatory response in the lumen of the intestine.
First, to determine whether curcumin treatment shows proinflammatory response by ER stress, the polarized intestinal cell line T84 was pretreated with or without curcumin for 24 hours followed by thapsigargin, as an ER stress inducer. We measured IL-8 mRNA expression level, a representative cytokine for NF-κB activation as a well-known of inflammatory pathway. As expected, thapsigargin induced the IL-8 mRNA level in a dose dependent manner (0, 1 and 3 µM). And, interestingly, treatment with curcumin reduced this IL-8 induction by thapsigargin in a dose dependent manner relatively (0, 0.1, and 1 µM) (see Fig. 4A).
Cholera toxin increases the ion current on the polarized T84 cell monolayer (measured by a real-time electrophysiology) due to raised cAMP level followed by ion channel opening, indicating the toxicity of pathogen (18). We therefore determined whether curcumin reduces pathogen toxicity, at least cholera toxin, in intestinal epithelial T84 cells. Toxicity is plotted on the Y-axis as a short circuit current, and time on the horizontal. As shown here, only the wild-type cholera toxin causes full toxicity when applied to T84 cells. Cells pretreated with curcumin showed half of the toxicity from the wild-type cholera toxin.
The polarized intestinal epithelial cell line T84 with/without pretreatment of curcumin for 24 hours was intoxicated with the wild-type cholera toxin, and the ion channel current (Isc, mA/cm2) was measured by electrophysiology in real time. As shown in Fig. 3B, a wild-type cholera toxin only started to induce toxicity (Isc, mA/cm2) 15 min after intoxication. However the intoxicated T84 cells pretreated with curcumin showed delayed toxicity starting from 45 min after intoxication, with almost half the level of full toxicity as compared to intestinal epithelial cells induced by treatment with a wild-type cholera toxin alone. As expected, neither buffer of the negative control, nor curcumin treatment itself showed toxicity (See Fig. 3B).
Collectively, curcumin delays the toxicity caused by the pathogen (at least cholera toxin) in the lumen and its maximum toxicity level is also reduced. The data in this study suggest the anti-toxicity action of curcumin.
Next, we determined whether curcumin affects ER stress response and inflammatory response induced by wild-type cholera toxin. BiP mRNA expression level, as a marker for ER stress (Fig. 3C), and IL-8 mRNA expression level as a marker for the inflammatory response (Fig. 3D), were measured on polarized intestinal Caco-2 cells pretreated with curcumin for 24 hours, followed by 3nM wild-type cholera toxin for another 24 hours.
Curcumin itself slightly induced the BiP mRNA level in Caco-2 cells (0, 0.1, 1, and 10 µM of curcumin), while it effectively inhibited the BiP mRNA level, which was induced by wild-type cholera toxin (0 nM versus 3 nM treatment of cholera toxin) in a dose dependent manner (Fig. 3C).
As shown in Fig. 3D, there was no induction of IL-8 mRNA level even with the highest concentration of curcumin (0 verse 10 µM of curcumin) in Caco-2 cells as well as T84 cells (Data not shown). Pretreatment with curcumin inhibited IL-8 induction by the wild-type cholera toxin in Caco-2 cells (Fig. 3D). This result suggests that curcumin treatment showed anti-inflammatory response in both T-84 and Caco-2 intestinal epithelial cells exposed to the pathogen, such as cholera toxin. Taken together, these data further indicate the protective function of curcumin in anti-inflammatory response by reducing ER stress against the pathogen in intestinal epithelial cells.
Furthermore, due to its potential toxicity, we wanted to confirm whether doses of curcumin used in this study affect the barrier function of polarized monolayer epithelial cells. When the cells were pretreated with curcumin for 24 hours before stimulation with 40 nM wild-type cholera toxin for another 24 hours, the tight junctions of the cells were intact, as shown with an anti- ZO-1 antibody, which is used as a marker for a tight junction (See Fig. 4A, right panels). In addition, we observed an actin skeleton of the cells using an anti-Phalloidin antibody in order to understand whether there is any change of the actin skeleton of the intestinal epithelial cells by curcumin (Fig. 4A, left panels). There was no change in the tight junction or actin skeleton by 1 µM of curcumin even in the presence of cholera toxin. Collectively, curcumin treatment itself is not harmful to the epithelial barrier even in the presence of inflammatory response to the pathogen.
The intestine consists of the small intestine and the large intestine, which is the major location of nutrient absorption as well as contact with pathogens/bacteria or their secreted toxins. The intestinal epithelial cell barrier is the first location of defense for innate immunity. In addition, intestinal immunity includes Peyer's patches (small intestine), lymphoid follicles, Mesenchyme lymph node (MLN), and colonic patches (large intestine) [14,15].
Microbial communities (microbiota) and pathogens influence the immune system and inflammation in the intestine. MUC2 is the most abundant protein of intestine mucus. Genetically engineered mice bearing MUC2 missense mutations develop chronic inflammation in their intestine. Mutant MUC2 mice induce stress in the endoplasmic reticulum (ER) and initiation of the unfolded protein response (UPR). The previous study showed evidence of ER stress and accumulation of inflammatory response [16]. NF-κB pathway is dominantly observed in inflammatory response of the intestine [15]. Receptor families such as IL-8, IL-11, and TNFα include activation of NF-κB signal transduction. The NF-κB refers to a group of transcription factors that regulate the expression of many genes encoding proteins which are broadly involved in immune and inflammatory response, cell death, cell cycle regulation, cell proliferation, and cell migration [17,18]. IKKα, IKKβ, and IKKγ (NF-κB essential modulator, NEMO) form the IKK complex, which phosphorylates IκBs [19,20]. The phosphorylation induces IκBα protein degradation, allowing movement of the free NF-κB to the nucleus, as a transcription factor. The effect of curcumin on inflammation is still unknown.
Several studies have reported evidence of a functional association of curcumin with intestine inflammatory disease, such as inflammatory bowel disease (IBD) [7]. Our data show that treatment with curcumin resulted in a reduction of BiP mRNA levels and IL-8 mRNA levels after cholera intoxication. Our study indicates that toxicity of cholera or pathogen might be controlled by curcumin treatment. Curcumin can reduce ER stress induction by thapsigargin or cholera toxin and thereby lead to reduction in inflammatory response (Fig. 4B). In addition, our study indicates that curcumin can also reduce the toxicity of pathogen. Using the electrophysiology assay, it was shown that curcumin treatment can reduce and delay the toxicity of wild-type cholera. Taken together, our study provides new evidence of the effect of curcumin in reducing ER stress and anti-inflammation in the intestine.
Finally, our data suggest that long-term or consistent intake of curcumin-containing food might prevent early bacterial or virus infection into the lumen of the intestine or reduce the degree of the infection by inhibiting the inflammatory response and an ER stress response from the intestinal epithelial barriers. In addition, it might imply that long-term intake of curcumin-containing food as a natural eco-biological ingredient might reduce the symptoms of inflammation in the intestine of IBD patients as a secondary treatment with few pharmaceutical side effects.
Figures and Tables
Fig. 1
Effect of curcumin showed reduction of ER stress in human intestinal epithelial cells.
Polarized human intestinal epithelia cell lines T84 (A and B) and Caco-2 (C and D) were treated with thapsigargin apically for 4 hours following 24 hour pretreatment with different concentrations of curcumin as indicated. RNA was extracted and hallmark of ER stress, BiP mRNA level was measured by qRT-PCR. The fold change of the thapsigargin treated group was normalized by curcumin treatment only (B and D) to eliminate basal difference from each concentration of curcumin. Open bars represent negative controls. Thaps: Thapsigargin, Data are expressed as mean ± SEM. *P < 0.05 by ANOVA (A and B), t-test (C and D).
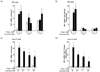
Fig. 2
Effect of curcumin on IRE1α activation in human intestinal epithelial cells.
Polarized human intestinal epithelia cell lines T84 (A and B) and Caco-2 (C) were treated with thapsigargin apically for 4 hours following 24 hour pretreatment with different concentrations of curcumin as indicated. mRNA was extracted and spliced form of XBP1 mRNA level (XBP1s), downstream target gene of IRE1α, was measured by qRT-PCR. The fold change of the thapsigargin treated group was normalized by curcumin treatment only (B) to eliminate basal difference from each concentration of curcumin. Open bars represent negative controls.

Fig. 3
Effect of curcumin on the defense mechanism against bacterial invasion in human intestinal epithelial cells.
Polarized human intestinal epithelia cell lines T84 (A and B) and Caco-2 (C and D) were treated with either thapsigargin for 4 hours (A) or 3 nM wild-type cholera toxin for 24 hours (B, C, and D) apically following 24 hour pretreatment with different concentrations of curcumin as indicated. RNA was extracted and the inflammatory response marker, IL-8 mRNA expression and ER stress marker, BiP mRNA expression level measured by qRT-PCR. Open bars represent negative controls. Thaps: Thapsigargin, Data are expressed as mean ± SEM. *P < 0.05 by ANOVA.
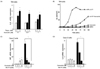
Fig. 4
Curcumin treatment displayed no change of the epithelial junction in human intestinal epithelial cells after intoxication.
(A) Polarized human intestinal epithelia cell line T84 cells were treated with 40 nM wild-type Cholera Toxin apically for 24 hours, respectively, following 24 hour pretreatment with 1 uM curcumin. The cells were fixed and stained with anti-ZO-1 antibody (green color for tight junction), anti-Phalloidin antibody (red color for actin skeleton), and DRAQ5 (blue color for nucleus). There was no change of the tight junction by curcumin. Arrows indicate the epithelial junction. (B) Proposed role of curcumin in anti-inflammatory response. wt CT: wild-type cholera toxins.

References
2. Cho JA, Lee AH, Platzer B, Cross BC, Gardner BM, De Luca H, Luong P, Harding HP, Glimcher LH, Walter P, Fiebiger E, Ron D, Kagan JC, Lencer WI. The unfolded protein response element IRE1alpha senses bacterial proteins invading the ER to activate RIG-I and innate immune signaling. Cell Host Microbe. 2013; 13:558–569.

3. Ji JL, Huang XF, Zhu HL. Curcumin and its formulations: potential anti-cancer agents. Anticancer Agents Med Chem. 2012; 12:210–218.

4. Kubota M, Shimizu M, Sakai H, Yasuda Y, Terakura D, Baba A, Ohno T, Tsurumi H, Tanaka T, Moriwaki H. Preventive effects of curcumin on the development of azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db obese mice. Nutr Cancer. 2012; 64:72–79.

5. Zeng Z, Zhan L, Liao H, Chen L, Lv X. Curcumin improves TNBS-induced colitis in rats by inhibiting IL-27 expression via the TLR4/NF-kappaB signaling pathway. Planta Med. 2013; 79:102–109.

6. Song WB, Wang YY, Meng FS, Zhang QH, Zeng JY, Xiao LP, Yu XP, Peng DD, Su L, Xiao B, Zhang ZS. Curcumin protects intestinal mucosal barrier function of rat enteritis via activation of MKP-1 and attenuation of p38 and NF-kappaB activation. PLoS One. 2010; 5:e12969.
7. Jian YT, Mai GF, Wang JD, Zhang YL, Luo RC, Fang YX. Preventive and therapeutic effects of NF-kappaB inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J Gastroenterol. 2005; 11:1747–1752.

8. Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, Dannenberg AJ. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis. 1999; 20:445–451.

9. Zhang M, Deng C, Zheng J, Xia J, Sheng D. Curcumin inhibits trinitrobenzene sulphonic acid-induced colitis in rats by activation of peroxisome proliferator-activated receptor gamma. Int Immunopharmacol. 2006; 6:1233–1242.

10. Bounaama A, Djerdjouri B, Laroche-Clary A, Le Morvan V, Robert J. Short curcumin treatment modulates oxidative stress, arginase activity, aberrant crypt foci, and TGF-beta1 and HES-1 transcripts in 1,2-dimethylhydrazine-colon carcinogenesis in mice. Toxicology. 2012; 302:308–317.

11. Epstein J, Docena G, MacDonald TT, Sanderson IR. Curcumin suppresses p38 mitogen-activated protein kinase activation, reduces IL-1beta and matrix metalloproteinase-3 and enhances IL-10 in the mucosa of children and adults with inflammatory bowel disease. Br J Nutr. 2010; 103:824–832.

12. Moon DO, Jin CY, Lee JD, Choi YH, Ahn SC, Lee CM, Jeong SC, Park YM, Kim GY. Curcumin decreases binding of Shiga-like toxin-1B on human intestinal epithelial cell line HT29 stimulated with TNF-alpha and IL-1beta: suppression of p38, JNK and NF-kappaB p65 as potential targets. Biol Pharm Bull. 2006; 29:1470–1475.

13. Lencer WI, Delp C, Neutra MR, Madara JL. Mechanism of cholera toxin action on a polarized human intestinal epithelial cell line: role of vesicular traffic. J Cell Biol. 1992; 117:1197–1209.

14. Glimcher LH, Lindvall O, Aguirre V, Topalian SL, Musunuru K, Fauci AS. Translating research into therapies. Cell. 2012; 148:1077–1078.

15. Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010; 140:859–870.

16. Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ, Adams R, Kato M, Nelms KA, Hong NA, Florin TH, Goodnow CC, McGuckin MA. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008; 5:e54.

17. DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012; 246:379–400.
19. Karin M. Tracking the road from inflammation to cancer: the critical role of IkappaB kinase (IKK). Harvey Lect. 2006-2007; 102:133–151.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download