Introduction
Skin thinning is a well-reported physical sign in dogs with hyperadrenocorticism (HAC) and a common side effect in dogs exposed to glucocorticoids [19232428]. Numerous clinicians have evaluated skin thickness of patients by using skinfold measurement (e.g., palpation, caliper) or skin biopsy methods. However, a skinfold measurement is poorly reproducible because it includes a varying proportion of subcutis, and the full skin biopsy is too invasive to measure skin thickness alone [2132]. Therefore, in humans, a recently developed high-resolution ultrasonographic approach has been widely used to measure the skin thickness in a non-invasive, simple, accurate and sensitive manner [120213435].
The range of mean skin thickness in dogs is 0.5 to 5 mm, and that range is too wide to be used for evaluation [22]. Furthermore, skin thickness is affected by a variety of physiological variables including anatomic site, breed, sex, age, and skin hydration [22]. Especially, the breed of dogs has been reported to have a significant effect on skin thickness, and therefore, previous studies of skin thickness without breed restriction have shown poor interpretation of the results [142238].
HAC is one of the most common endocrine diseases in dogs and is diagnosed and differentiated by hormonal assays such as adrenocorticotropic hormone (ACTH) stimulation test or the low-dose dexamethasone suppression test (LDDST). However, the hormone assay is expensive and tedious, and clinicians evaluate HAC by using a variety of supplementary screening tests [3824]. Ultrasonographic assessment of adrenal gland is a popular method among the various screening tests [212]. If the skin thickness of a HAC patient is significantly different from that of a normal dog and the difference between them can be reliably evaluated by ultrasonography, the usefulness of ultrasonography in screening of HAC will be further increased.
In this study, we determined the normal range of skin thickness in small breed dogs and determined the correlation between skin thickness and various physiological variables. We compared normal groups with several groups that are likely to have affected skin thickness and evaluated the possibility of differentiating HAC patients by evaluating skin thickness.
Materials and Methods
Animals
This retrospective study was performed in small breed dogs (< 15 kg) that underwent abdominal ultrasonographic examinations at the Veterinary Medical Teaching Hospitals of Gyeongsang National University from October 1, 2010 to September 30, 2017. The 137 included dogs were included and divided into three groups: normal dogs, dogs with spontaneous HAC, and dogs receiving prednisolone (PDS). All procedures were approved by the Institutional Animal Care and Use Committee of Gyeongsang National University (GNU), and the dogs were handled according to the Guidelines for Animal Experiments (GAR-101118-X0010) of GNU. Dogs with a recent history of primary skin disease or treatment with topical agents (e.g., spray, ointment) were excluded.
Normal dogs
Based on history, physical examination, complete blood count (CBC), routine serum chemistry analyses, and imaging results, 71 dogs were deemed clinically normal. The following parameters were evaluated on a priority basis as exclusion criteria: physical signs (polyuria, polydipsia, abdominal distension, alopecia, thin skin, hyperpigmentation), imaging observations (hepatomegaly, adrenomegaly), increased alkaline phosphatase (ALP) activity exceeding 150 U/L, and cholesterol concentration exceeding 8 mmol/L. The 71 dogs included the following breeds: Beagle (n = 21), Maltese (n = 13), Shih Tzu (n = 10), Pomeranian (n = 9), Toy Poodle (n = 8), Yorkshire Terrier (n = 7), and Pekinese (n = 3). The group included 11 intact males, 26 castrated males, 18 intact females, and 16 spayed females. Their ages ranged from 4 months to 17 years, and the group's average weight was 6.58 ± 3.61 kg (range, 1.2–15 kg).
Dogs with spontaneous hyperadrenocorticism
Thirty dogs were tentatively diagnosed with HAC based on history, clinical signs, physical examination, CBC, routine serum biochemical analysis, and imaging results (hepatomegaly and adrenomegaly). The diagnosis of HAC was confirmed by the presence of (1) an exaggerated serum cortisol level exceeding 20 µg/dL, after 1 h of ACTH stimulation, and (2) improvement after treatment for HAC with trilostane or lack of suppression of serum cortisol levels above 1.5 µg/dL, after 8 h of low-dose dexamethasone administration. The 30 dogs included the following breeds: Shih Tzu (n = 8), Maltese (n = 6), Miniature Schnauzer (n = 6), Toy Poodle (n = 3), Yorkshire Terrier (n = 2), Pomeranian (n = 1), Pekinese (n = 1), Chihuahua (n = 1), Pug (n = 1), and Dachshund (n = 1). This group included 9 intact males, 1 castrated male, and 20 intact females. The group's age ranged from 3 to 17 years, and its average weight was 5.5 ± 1.87 kg (range, 2.1–8.6 kg). Ten of these dogs were treated with trilostane for at least one month (411 ± 490 days; range, 34–1,381 days) before reassessment after treatment.
Dogs receiving prednisolone
Thirty-seven dogs received oral PDS for a period of at least 1 month (483 ± 550 days; range, 28–1,843 days) and at various dosages (0.25–2 mg/kg twice daily) for a variety of disorders, including MUE (n = 13), immune-mediated disease (n = 7), brain tumor (n = 5), intervertebral disc disease (n = 4), respiratory disease (n = 3), hydrocephalus (n = 3), chronic otitis (n = 1), and chronic hepatitis (n = 1). The 37 dogs included the following breeds: Maltese (n = 10), Yorkshire Terrier (n = 6), Shih Tzu (n = 5), Pomeranian (n = 5), Toy Poodle (n = 3), Chihuahua (n = 3), Miniature Schnauzer (n = 1), Pekinese (n = 2), French Bulldog (n = 1), and Dachshund (n = 1). This group included 18 intact males, 1 castrated male and 18 intact females. The group's age ranged from 3 to 16 years, and its average weight was 4.6 ± 2.44 kg (range, 1.29–11.3 kg).
Equipment and measurement
Two ultrasound scanners (Arietta 70 [Hitachi Aloka Medical, Japan] and Xario SSA-660A [Toshiba, Japan]) equipped with high-frequency (12 MHz) linear-array transducers were used for abdominal ultrasonography. Three B-mode digital images corresponding to cranial, left and right abdomen for each patient were used for measurement. Serial data were collected and reviewed by one of the experienced investigators. The measurements were performed on a Digital Imaging and Communications in Medicine workstation using an electronic caliper. Mean values were obtained by repeating measurements three times in each region. The same data were re-evaluated after one month under the same conditions to evaluate intraobserver reliability of the measurement.
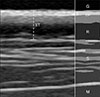
Ultrasonographic appearance of the normal skin in dogs was identified as a regular pattern characterized by several distinct layers (Fig. 1). The first hyperechoic line identified below the gel represented the entry echo, which is the acoustic interface created when ultrasound penetrates between the surface of the epidermis and the gel. The dermis is located beneath the epidermis and appears as a thicker and less echogenic line than that of the epidermal entry echo. A clear distinction between the dermis and the epidermis may be difficult because the entry echo may obscure the boundary. The dermis is represented as a two-layered structure if the reticular dermis under the papillary dermis is more hypoechoic due to the abundant interstitial fluid. The distance between the epidermal entrance and the end of the dermis was measured. Subcutis was not included in the skin thickness due to the uncertain boundary with the muscle.
Statistical analysis
All statistical analyses were performed with IBM SPSS Statistics for Windows (ver. 19.0.0; IBM, USA) and the mean values of the 3 abdominal regions were assessed. The intraclass correlation coefficient was calculated to evaluate intraobserver reliability in measurements of skin thickness. One-way ANOVA or Pearson correlation was used to determine the relationship between skin thickness and physiological variables (abdominal region, breed, weight, sex, age) in normal dogs. One-way ANOVA followed by the post hoc Scheffé's test was used to compare the mean value of skin thickness in each group. A paired t-test was performed after a normality test to determine changes in skin thickness after treatment with trilostane in 10 HAC patients. The receiver operating characteristic (ROC) curve was used to compare the sensitivity and specificity of the values used to differentiate the normal group from the HAC group. Values of p under 0.05 were considered significant.
Results
Intraobserver reliability of the measurement
The interclass coefficients for intraobserver reliability were 0.94, 0.93, and 0.83 for each group (normal dogs, dogs with HAC, and dogs treated with PDS). The reproducibility of the ultrasonographic measurements for skin thickness was excellent in all groups.
Normal range of skin thickness and correlation with variables
There was no significant difference in skin thickness among the abdominal regions in the normal group (p = 0.718). The mean skin thickness was 1.03 ± 0.25 mm (mean ± SD) and the 95% confidence interval was 0.97 to 1.09 mm. There was no correlation between skin thickness and breed (p = 0.272). A weak positive correlation existed between skin thickness and body weight (r = 0.254, p = 0.033). There was no difference in skin thickness according to sex (p = 0.198), and there was no correlation between skin thickness and age (r = 0.164, p = 0.172).
Comparison of skin thickness among groups
Mean ± SD (range) skin thicknesses of the 3 groups (normal dogs, dogs with HAC, and dogs receiving PDS) were 1.03 ± 0.25 mm (0.43–1.67 mm), 0.74 ± 0.26 mm (0.43–1.37 mm), and 0.57 ± 0.18 mm (0.27–1.10 mm), respectively. Both the HAC and PDS groups had significantly thinner skin than that in the normal group (p = 0.001, p = 0.001, respectively), and the difference in skin thickness between the normal and PDS groups was greater than that between the normal and HAC groups (Fig. 2). Seven of the 10 HAC patients treated with trilostane showed increased skin thickness and three dogs had similar thickness to that at pre-treatment. The mean skin thickness in the 10 HAC patients was 0.70 ± 0.23 mm (0.56–1.30 mm), while the mean skin thickness of patients treated with trilostane was 0.97 ± 0.29 mm (0.67–1.70 mm) (Fig. 3). The thickness of the skin before treatment was significantly greater than that after treatment (p = 0.03), with an average increase of 39%.
Differentiation between normal dogs and HAC patients
Based on the results of the ROC curve test, the area under the ROC curve was 0.807. Measurement of skin thickness was used to accurately differentiate between normal and HAC patients (Fig. 4); sensitivity was 76% and specificity was 73% when a skin thickness of 0.83 mm or less was used as the cutoff value.
Discussion
Measurement of skin thickness by using ultrasonography has been reported as a reproducible method in humans [135] and dogs [14]. Differences in the tissue constituting the dermis and subcutis create a clearly recognizable interface on ultrasound image [1016], and the overall intraobserver reliability of measurement was excellent in this study.
In two previous studies [1314], the abdominal skin thicknesses of normal dogs obtained by using ultrasonography were 1.93 ± 0.59 mm and 2.24 ± 0.58 mm, nearly twice the value of the normal group in this study. Large dog breeds seem to have a thicker skin than that in small dog breeds, and the two previous studies contained a large proportion of large dog breeds [1314], in contrast to the dogs in this study. Another three studies measured abdominal skin thickness by using skin biopsy. In those studies, skin thickness in normal dogs was reported as 1.3 ± 0.34 mm [25], 0.95 ± 0.11 mm [4], and 0.62 mm [27]. The results in the first two studies [425] were similar to the results of this study, probably because they measured beagles, a small dog breed. The results of another study [27] showed significantly thinner skin than the mean skin thickness of this study, but the breed was not identified. A biopsy is one of the most accurate methods for the measurement of skin thickness but there is a possibility of altered thickness during the preparation of tissues for histological examination [142935].
Although abdominal skin thickness tended to be less than that in other regions, the abdomen was considered as an appropriate site for the evaluation of skin thickness in this study because of its clinical accessibility and its limited influence on hydration status [1322]. As there was no significant difference in skin thickness between the cranial, right, and left regions of the abdomen, the mean value was used for evaluation.
Significant effects of breed on skin thickness have been reported in previous studies [142238], but were not detected in this study. The normal range of skin thickness in this study showed a relatively low standard deviation compared to those in previous reports in which dog breed was not restricted [14]. This suggested that restriction of the breed is an essential requirement for the study of skin thickness in dogs.
Despite the significant correlation between breed and body weight in dogs [9], no significant correlation was detected in a previous study (r = 0.310, p = 0.122) [14], and a weak correlation was detected in this study (r = 0.254, p = 0.033). It is estimated that the biophysical parameters of the skin that affect skin thickness, including hydration, sebum, and transepidermal water loss, are determined by breed rather than by body weight [38].
In humans, male skin is about 10% thicker than the skin of female [1533]. However, in this study, there was no significant difference in skin thickness according to sex, a result that is similar to that in a previous study [38].
It has been reported that skin thickness can be decreased in older humans, aged 60 to 70 years, due to reduced levels of interstitial fluid, proteins, and collagen in the skin [33]. However, the effect of age on skin thickness may vary depending on body region and photodamage, and the relationship between age and skin thickness is unclear [1736]. One study [38] reported a significant negative correlation between age and skin thickness in dogs, but no such correlation was detected in this study. It is presumed that the interpretation of results without distinction of breed in previous study may have resulted in such a difference.
It has been reported that both total skin collagen and skin thickness were decreased in patients with HAC [6]. In one study, skin thinning was observed in 60% of dogs with HAC, and their mean skin thickness was 25% less than normal [27]. In this study, similar to results in previous reports, the HAC group showed an average 29% lower skin thickness than that in the normal group. However, skin thinning was not identified in all patients in the HAC group, which may be due to individual differences in severity of disease or cortisol sensitivity [5].
Both topical and oral glucocorticoid therapies induce skin thinning, and the pathological changes in skin thinning are similar to that of HAC [63132]. The degree of skin thinning varies depending on the potency, duration, and frequency of application, but even low-potency topical glucocorticoids induce skin thinning [1932]. In humans, it has been reported that skin is 20% to 33% thinner in patients taking oral PDS or prednisone, and skin is about 16% to 25% thinner in patients applying topical glucocorticoids [1120263437]. Skin thinning is also reported as the most common side effect in a dog with iatrogenic HAC [5718]. In the present study, the skin in the PDS group was about 45% thinner than normal and suggested a more significant difference than that previously reported. This difference in skin thickness is probably due to the long-term use of high-dose PDS at levels of immunosuppression for underlying disease in most patients belonging to the PDS group. Strong glucocorticoids have been reported to cause not only a high degree of skin thinning but also irreversible skin thinning [1932].
In humans, the skin thinning is known to be reversible if the primary cause is resolved. Skin thinning associated with glucocorticoid use was reversed to its previous condition upon treatment cessation, and there was improvement of abnormal skin conditions in 60% to 90% of dogs with HAC after treatment with trilostane [1923262839]. Similar to previous reports, improvement in skin thinning was observed in 70% of patients treated with trilostane in this study.
Skin thinning is a well-known clinical symptom in HAC patients. However, systematic evaluation is difficult because of the lack of clear evaluation criteria. However, in small dog breeds, quantitative evaluation of skin thickness may be a possible screening test for HAC because normal variation in skin thickness was established and the HAC group showed a significant difference from normal in this study. However, in addition to HAC, various conditions that affect skin thickness including PDS, trilostane, and other variables were not investigated in this study. For example, there is a possibility that malnutrition or hydration status may also affect skin thickness [1330]. A larger than expected overlap between groups was observed in this study, and the accuracy of differentiation between HAC patients and normal dogs was further reduced.
This study's limitations are as follows. The LDDST was not performed in all patients. However, the purpose of excluding patients with iatrogenic HAC from the HAC group and of monitoring the HAC patients treated with trilostane would have been accomplished via the ACTH test [324]. The study strategy accurately identified HAC patients by excluding patients treated with doses that were not generally consistent with HAC in the various screening tests. Alterations in pressure may change skin thickness depending on the scan procedure; however, this retrospective study comprised various patient groups that had skin thickness measured without consideration of the pressure used. The results from each group may reflect the tendencies of certain breeds because breed distribution was not exactly similar in each group. Another possibility was that the breeds included in this study were insufficient to represent small breed dogs.
There are large individual differences in skin thickness of dogs due to the effect of various physiological variables. However, if the assessed dogs are restricted to those from small dog breeds, measurement of skin thickness by using ultrasonography is likely to provide clinical information that may be used to differentiate HAC patients from normal dogs. However, exposure to PDS, trilostane, and other conditions may have significant effects on skin thickness, and such differentiation should not be evaluated based on skin thickness alone.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download