Abstract
The aim of the present study was to investigate the diagnostic accuracy of ultrasonography in the detection of mild pneumothorax using computed tomography (CT) in dogs. Nine adult healthy beagles were included in the study. A thoracic tube was inserted into pleural space at the left thoracic wall, and each dog underwent the examinations in the order of CT, lung ultrasonography, and radiography before the infusion of room air into the pleural space. Two, 3, and 5 mL/kg infusions of room air were sequentially introduced into the pleural space and CT, lung ultrasound, and radiography examinations were performed. Sonographic signs included A-lines, stratosphere, lung sliding, lung point, lung pulse, and reverse sliding signs. Radiographs were evaluated for the absence or presence of a pneumothorax. Lung ultrasound results were more accurate than radiography results for the detection of mild pneumothorax. The overall sensitivity of the sonographic reverse sliding sign was higher than that of other sonographic signs, and its specificity was 100% for detection of mild pneumothorax. Thus, the reverse sliding sign is useful when using lung ultrasonography for diagnosis of mild pneumothorax.
Transthoracic lung ultrasonography is emerging as a diagnostic examination approach for a variety of lung diseases [182022] and in the detection of pneumothorax [2731] in human. A mild pneumothorax is not usually life-threatening, but delayed diagnosis can cause disease associated with resultant circulatory and respiratory compromise in unstable patients [8]. Tension pneumothorax is an emergency condition, and because tension pneumothorax can lead to impairment of blood circulation and respiration, early diagnosis and treatment are required [12]. The diagnosis of pneumothorax is identified by using thoracic radiography, but thoracic radiography is a relatively insensitive and unreliable diagnostic tool [5]. In contrast, computed tomography (CT) scanning is deemed the gold standard for evaluation of pneumothorax [1829].
Theoretically, air-filled lung parenchyma cannot be visualized by ultrasound [19]. The potential for ultrasound to examine the lung is classically thought to be limited since air is considered an insurmountable obstacle [17]. Ultrasonographic images are thus exclusively composed of artefacts. Recently, some ultrasonographic features have been confirmed in humans in the diagnosing of pneumothorax, such as the A-line sign [13], stratosphere sign [16], lung slide [16], lung point [14], and lung pulse [28] features. In normal lung, respiration-dependent motion of the lung surface is visualized via transthoracic lung ultrasound and is called lung gliding or lung sliding [212730]. The presence of lung slide, a to-and-fro motion visible at the pleura that occurs with respiration, is an important feature in normal air-filled lungs [10131621]. This is one of the dynamic signs of the lung and is identified by horizontal motion along the pleura in sonography [7]. A lung slide image can be objectified by using an M-mode as the superficial parietal lines are motionless and appear in a horizontal pattern, whereas the area deep in the pleural interface appears as a “sand-like pattern” as the motion of the pleural line is reflected all over this area. This feature is called the seashore sign [311]. In a pneumothorax, the abnormal air in the pleural cavity separates the parietal and visceral pleura, rendering the visceral pleura invisible. Because of this, lung slide cannot be confirmed in M-mode. The complete absence of dynamics results in superimposing strictly horizontal lines, called the barcode or stratosphere sign [31116]. The lung point is identified at the boundary between normal lung and collapsed lung in the pneumothorax. In the M-mode, the lung point is identified by the appearance of alternating lung sliding (seashore sign) and absent sliding (stratosphere sign) during respiration [10]. Lung pulse is a vertical motion of the pleura in synchrony with the cardiac rhythm [28]. Air in the pleural space prevents transmission of movement through the lung to the parietal pleural interface. If a lung pulse is identified, pneumothorax can be excluded [28]. However, if lung pulse is not identified, a pneumothorax cannot be diagnosed. Thus, this sign is only of significance if it is present [1015]. Features of the reverse sliding sign are identified as: (a) patterns suggestive of pneumothorax, (b) movement in the opposite direction of sliding, and (c) appearance of this sign on inspiration.
It is difficult to use thoracic radiography to diagnose a small amount of air in the mild pneumothorax. In addition, CT scanning cannot be performed routinely, is expensive, and it requires deep sedation or anesthesia. Because transthoracic lung ultrasound can be performed easily and quickly, it can be used in the diagnosis of pneumothorax in trauma [425], after lung biopsy [2326], and in ventilated patients [1617]. However, previous studies have not reported on ultrasonographic features in the detection of mild pneumothorax in dogs. The aim of this study was to investigate the diagnostic accuracy of ultrasonography and to identify the accuracy of ultrasonographic features in the diagnosis of mild pneumothorax using CT in dogs.
All procedures were approved by the Institutional Animal Care and Use Committee of Gyeongsang National University (GNU), and the dogs were maintained according to the Guidelines for Animal Experiments (GNU-161031-D0063) of GNU. Nine healthy beagles were included in the study (mean weight, 11.1 kg [range, 8–16 kg]; mean age, 6.2 years [range, 4–8 years]; seven males and two females). The dogs had no evidence of cardiovascular or respiratory diseases on physical, thoracic radiography, echocardiography, and thoracic CT examinations.
The dogs were fasted for 12 h before anesthesia. Anesthesia was induced by propofol (6 mg/kg, intravenous; Myungmoon, Korea) and maintained with isoflurane (Hana Pharm, Korea) in oxygen via endotracheal intubation. During the experiment, all dogs remained anesthetized. The dogs were positioned in right lateral recumbency for insertion of a thoracic tube at the left thoracic wall. A flexible extension tube (extension tube; Shinchang Medical, Korea) was inserted into the cutaneous tissue at the tenth intercostal space and was advanced into the pleural space at the eighth intercostal space. The tube was connected to a three-way stopcock. The location and placement of the chest tube were confirmed via CT in the sternal recumbency position. The dogs underwent the examinations in the order of CT, lung ultrasound, and thoracic radiography before room air was injected into the pleural cavity. Subsequently, room air (2 mL/kg) was injected into the pleural cavity in the sternal recumbency position and CT, lung ultrasound, and thoracic radiography examinations were performed. Next, an additional 3 mL/kg (total infusion of 5 mL/kg) of room air were introduced into the pleural cavity and the same imaging protocol was followed. This was followed by introducing a further 5 mL/kg (total of 10 mL/kg) of room air and the same imaging protocol was followed. At the end of the experiment, the pleural air was evacuated from the pleural space. All dogs were confirmed to have no pneumothorax by performing thoracic radiography after 24 h.
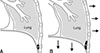
The examinations were performed on a commercially available two-channel CT scanner (Somatom Emotion; Siemens Medical Systems, Germany). CT images were obtained with full inspiration of the lung with the dogs in the sternal recumbency position. The acquisition parameters were as follows: 3 mm collimation and 50 mA and 110 kV tube voltage. The CT scans were evaluated at the window width of +1,200 Hounsfield units (HU) and a window level of −600 HU. The CT was used to confirm the absence or presence of a pneumothorax (Fig. 1).
All dogs underwent transthoracic examination by using an ultrasound scanner (Arietta 70; Hitachi Aloka Medical, Japan) equipped with 2 to 12 MHz linear probe settings such as tissue harmonics and spatial compound functions turned off. Such settings may eliminate artefacts and could impede diagnosis. The hair was clipped from the examination area and gel was used. The scanning was performed immediately after the CT scan by an experienced sonographer (T.S. Hwang) using the intercostal spaces with the dog in the sternal recumbency. For pneumothorax evaluation, scanning was performed in eight regions: right (Rt.) cranioventral, Rt. craniodorsal, Rt. caudoventral, Rt. caudodorsal, left (Lt.) cranioventral, Lt. craniodorsal, Lt. caudoventral, and Lt. caudodorsal. The shoulder joint was established as the measure between ventral and dorsal regions and the sixth intercostal space as the measure between the cranial and caudal regions (Fig. 2). The eight regions were identified by using horizontal scans and the probe was moved with a sliding scan. Dynamic as well as static sonography features were identified for each dog. The static sonographic sign was the A-line (Fig. 3). Dynamic sonography features included the lung slide (panel A in Fig. 4), stratosphere sign (panel B in Fig. 4), lung pulse (panel C in Fig. 4), lung point (panel D in Fig. 4), and reverse sliding sign (Figs. 5 and 6).
Thoracic radiography included ventrodorsal, left, and right lateral views in all dogs. The radiographs were used to confirm the absence or presence of a pneumothorax. Signs of pneumothorax on thoracic radiographs were defined as (a) increased opacity of collapsed lobe and retracted lung margin, (b) elevation of the cardiac silhouette from the sternum, and (c) no lung markings visible peripherally.
Statistical analysis was performed by using a commercially available statistical analysis program (IBM SPSS Statistics ver. 19.0; IBM, USA). The data were arranged in 2 × 2 tables representing true and false positives and true and false negatives. CT, lung ultrasound, and thoracic radiography images were identified and characterized by the presence or absence of a pneumothorax. The thoracic radiography and thoracic ultrasonography were compared with the results of CT. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of transthoracic lung ultrasonography and radiography were calculated. For the different pneumothorax volumes, the sensitivity, specificity, PPV, and NPV of transthoracic lung sonographic signs (lung slide, lung pulse, lung point, and reverse sliding sign) were calculated. We analyzed the receiver operating characteristic (ROC) curves and the area under the curves (AUC). Values of more than 0.5 and a p value of less than 0.05 were considered to indicate statistical significance, respectively.
In total, 108 thoracic radiographic images, 288 thorax sonographic images, and 288 videos were obtained from the nine dogs. The pooled sensitivity, specificity, NPV, PPV, and AUC for diagnosis of pneumothorax with lung ultrasound and thoracic radiography are shown in Table 1. The results show that transthoracic lung ultrasound was more sensitive and specific than thoracic radiography in diagnosing pneumothorax. Pooled sensitivity and specificity were 88.8% and 88.8%, respectively, for ultrasonography and 66.6% and 88.8%, respectively, for thoracic radiography. The ROC curve AUC was 0.939 for transthoracic lung ultrasonography. For thoracic radiography, the ROC curve AUC was 0.825.
For the different pneumothorax volumes (2, 5, and 10 mL/kg), the sensitivity, specificity, PPV, and NPV of the lung sonographic signs (lung slide, lung pulse, lung point, and reverse slide sign) were calculated (Table 1). The sensitivity and PPV for the 5 and 10 mL/kg volumes for the absence of lung slide and lung pulse were considerably higher than those for the 2 mL/kg pneumothorax volume. The overall sensitivity and specificity for the absence of lung sliding were 44.4% and 88.8%, respectively, and the overall sensitivity and specificity for the absence of lung pulse were 44.4% and 77.7%, respectively. As the volume of the pneumothorax increased, the sensitivity of the lung point also increased. However, the overall sensitivity of the lung point was low (33.3%). The lung point specificity was 100% for the diagnosis of pneumothorax. The reverse sliding sign was only confirmed in the caudodorsal thorax and had a specificity of 100% for the diagnosis of pneumothorax. As the volume of the pneumothorax increased, the sensitivity of the reverse sliding sign was observed to decrease. However, overall sensitivity was higher (85.1%) than that for other sonographic signs.
In our study, transthoracic lung ultrasonography showed a higher sensitivity than thoracic radiography (88.8% vs. 66.6%) and also showed higher PPV (96.0% vs. 94.7%) and NPV (72.7% vs. 47.0%). In a human study, the sensitivity of transthoracic lung ultrasonography and thoracic radiography were 78.6% and 39.8%, respectively [2]. The pooled specificity for transthoracic lung ultrasonography and thoracic radiography in the reported study were 98.4% and 99.3%, respectively. The AUC for lung ultrasound and radiography were 0.980 and 0.959 [2]. In another study, on a series of 184 patients who underwent lung ultrasound after a percutaneous needle biopsy, lung ultrasonography was compared with thoracic CT and thoracic radiography [9]. The results revealed identification of pneumothorax in 46 patients by thoracic CT, in 44 patients by lung ultrasound (without a false positive), and in 19 patients by thoracic radiography. In that study, lung ultrasonography sensitivity (95.6%) and specificity (100%) were slightly higher than our results. Chung et al. [6] compared the detectability of high-resolution ultrasound and bedside radiography for pneumothorax in 97 patients. Among the 97 patients, 35 patients were identified as pneumothorax by thoracic CT after percutaneous needle biopsy and aspiration. The sensitivity for detection of pneumothorax was 80% and 47% in high-resolution ultrasound and thoracic radiography, while the specificity was 94% and 94%, respectively, and the diagnostic accuracy was 89% and 77%, respectively. Soldati et al. [2425] studied two series of patients with polytrauma and blunt thoracic trauma for the diagnosis of pneumothorax by using transthoracic lung ultrasonography. The results were compared with those from thoracic CT and thoracic radiography, and they concluded that lung ultrasonography could detect pneumothorax undiagnosed by thoracic radiography and that lung ultrasonography was as accurate as thoracic CT. These previous studies have identified that transthoracic lung ultrasonography is an accurate modality compared to thoracic radiography in pneumothorax. Our study also showed that transthoracic lung ultrasound is more accurate than thoracic radiography for the detection of mild pneumothorax. The sensitivity of our study (88.8%) was similar to that in previous pneumothorax studies (78.6–95.6%). The specificity (88.8%) was slightly lower than that in previous pneumothorax studies (94–100%). The result is considered to be due to the small pneumothorax volume compared those in previous studies. However, the specificity of 88% is considered to be high. Therefore, we considered that transthoracic lung ultrasonography is useful in lung ultrasonography for the diagnosis of mild pneumothorax.
The sensitivity and PPV of the 5 and 10 mL/kg volumes for the absence of lung slide and lung pulse were considerably higher than those for the 2 mL/kg pneumothorax volume. We suggest that it is difficult for a sonographer to find signs in a small volume of pneumothorax. When the volume of the pneumothorax increased, the sensitivity of the lung point also increased, but the overall sensitivity was low. Lung point had a specificity of 100% for the diagnosis of pneumothorax. Similarly, a previous study revealed that the lung point had a specificity of 100% in the detection of pneumothorax [14]. Lung point has been referred to as an all-or-nothing sign [17]. However, if total lung collapse occurs with severe pneumothorax, the lung point may not be identified [14].
When the volume of the pneumothorax increased, the sensitivity for the reverse sliding sign showed a decreased sensitivity. However, a higher overall sensitivity for the reverse sliding sign was observed. The reverse sliding sign had a specificity of 100% in the detection of a mild pneumothorax. However, the reverse sliding sign is also an “all-or-nothing” pattern in the caudodorsal thorax. In this study, the reverse sliding sign was confirmed to move in the opposite direction to that of lung sliding in the caudodorsal thorax during inspiration. A minimal volume of air within the pleural space can remain in the caudodorsal thorax in the adjacent rib in a sternal recumbency patient, and a sonographer may visualize an A-line and an absence of lung slide in this area. During inspiration, the reverse sliding sign is observed where the point of escape of the trapped pleural air is found. In addition, the half image under the transducer footprint shows motion of the A-line in the caudal direction, while the other half of the image shows the opposite direction of motion in the caudodorsal thorax. Thus, the reverse sliding sign may be explained based on the escape of trapped pleural air because of increase in the size of the thoracic cavity during inspiration.
Lung ultrasonography-based diagnosis of a pneumothorax is normally a “rule out” type of examination. The absence of a lung pulse or lung slide cannot confirm a pneumothorax, but the presence of both the lung pulse and the lung slide can rule out a pneumothorax [1015]. A previous report suggested that in the absence of either lung pulse or lung slide, the other sign should be identified carefully before diagnosing a pneumothorax [1317]. The lung point is rarely found, but it is a specific sign that confirms the presence of a pneumothorax [815]. Additionally, the reverse sliding sign could improve the accuracy of pneumothorax diagnosis based on ultrasonography. Regardless, despite its security, simplicity, and rapidity, lung ultrasound has limitations in the diagnosis of pneumothorax such as in patients with cutaneous emphysema, calcifications, and thoracic dressings [8].
There are some limitations to this study. First, our study was performed in a small number of the dogs with mild pneumothorax; thus, the results should be confirmed in a large number of the dogs and in those with severe pneumothorax. Second, all dogs underwent the examinations in the order of CT, lung ultrasound, and thoracic radiography examination after the induction of a pneumothorax. In the current study, CT and transthoracic lung ultrasound were performed in an average of three minutes and seven minutes, respectively, after pneumothorax induction. The thoracic radiography was performed after a slight time lag after the induction of pneumothorax, thus there was time available for a slight decrease in pneumothorax volume.
In conclusion, our study has shown that lung ultrasonography is more sensitive than thoracic radiography for detecting mild pneumothorax in dogs. In particular, the reverse sliding sign was more sensitive than the other ultrasonography signs, and it had 100% specificity for detecting mild pneumothorax. Thus, the reverse sliding sign is useful in lung ultrasonography for the diagnosis of mild pneumothorax. We conclude that clinician-performed transthoracic lung ultrasound is a reliable examination method for the diagnosis of mild pneumothorax.
Figures and Tables
Fig. 1
Diagnosis of pneumothorax by using computed tomography (CT). (A) Pneumothorax (arrows) of the cranial thorax on axial CT image. (B) Pneumothorax (arrows) of the caudal thorax on axial CT image.
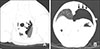
Fig. 2
Transthoracic lung ultrasound scan areas. Diagram illustrating location criteria used for dividing each dog's hemithorax into four quadrants, using the shoulder joint as the limit between the ventral and dorsal quadrants and the sixth intercostal space as the limit between the cranial and caudal quadrants.

Fig. 3
Normal lung transthoracic ultrasound image. Horizontal A-lines are present in healthy dogs. R, rib; P, pleural surface; A, reverberation artefact of the pleural surface.
Fig. 4
The time-motion mode objectifies the lung slide (A), stratosphere (B), lung pulse (C), and lung point (D) signs. (A) Below the pleural line, lung sliding generates a homogeneous granular pattern. (B) Parallel horizontal lines above and below the pleural line, resemble a ‘barcode’ and indicates the absence of lung sliding. (C) Vertical movement of pleural lines synchronous to the cardiac rhythm (arrows). (D) The sudden inspiratory appearance of a granular pattern beneath the pleural line (arrow).
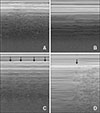
Fig. 5
The two-dimensional mode objectifies normal lung (A and B) and reverse sliding sign (C and D) in ultrasound images. (A) During expiration, horizontal A-lines (arrowhead) were present in healthy dogs. (B) During inspiration, A-lines showed lung sliding in the caudal direction (arrowhead). (C) During expiration, caudal lung lobe (arrowhead) was present. A minimal amount of trapped pleural air may remain in the caudodorsal thorax in the area of the adjacent rib (arrow). The trapped air visualized as A-line in this area. (D) During inspiration, the trapped pleural air escapes because of an increase in the size of the thoracic cavity. The half image under the transducer shows lung slide in the caudal direction (arrowhead), while the other half of the image shows the sliding sign in the opposite direction in the left caudodorsal thorax (arrow). Asterisks, left lateral hepatic lobe.
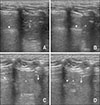
Fig. 6
A theoretical explanation of the reverse sliding sign. (A) During expiration, a minimal amount of pleural air (asterisk) may remain in the caudodorsal thorax in the area of the adjacent rib in sternal recumbency dogs. (B) During inspiration, trapped pleural air (asterisk) escapes because of increase in the size of the thoracic cavity; that is, the lung is inflated in the caudal direction during inspiration, while trapped air escapes in a direction opposite to that of lung sliding in the caudodorsal thorax.
References
1. Abdalla W, Elgendy M, Abdelaziz AA, Ammar MA. Lung ultrasound versus chest radiography for the diagnosis of pneumothorax in critically ill patients: a prospective, single-blind study. Saudi J Anaesth. 2016; 10:265–269.

2. Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care. 2013; 17:R208.

3. Barillari A, Kiuru S. Detection of spontaneous pneumothorax with chest ultrasound in the emergency department. Intern Emerg Med. 2010; 5:253–255.

4. Blaivas M, Lyon M, Duggal S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med. 2005; 12:844–849.

5. Chiles C, Ravin CE. Radiographic recognition of pneumothorax in the intensive care unit. Crit Care Med. 1986; 14:677–680.

6. Chung MJ, Goo JM, Im JG, Cho JM, Cho SB, Kim SJ. Value of high-resolution ultrasound in detecting a pneumothorax. Eur Radiol. 2005; 15:930–935.

7. De Luca C, Valentino M, Rimondi MR, Branchini M, Baleni MC, Barozzi L. Use of chest sonography in acute-care radiology. J Ultrasound. 2008; 11:125–134.

8. Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest. 2011; 140:859–866.

9. Garofalo G, Busso M, Perotto F, De Pascale A, Fava C. Ultrasound diagnosis of pneumothorax. Radiol Med. 2006; 111:516–525.

10. Husain LF, Hagopian L, Wayman D, Baker WE, Carmody KA. Sonographic diagnosis of pneumothorax. J Emerg Trauma Shock. 2012; 5:76–81.

11. Johnson A. Emergency department diagnosis of pneumothorax using goal-directed ultrasound. Acad Emerg Med. 2009; 16:1379–1380.

12. Leigh-Smith S, Harris T. Tension pneumothorax: time for a re-think? Emerg Med J. 2005; 22:8–16.
13. Lichtenstein D, Mezière G, Biderman P, Gepner A. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999; 25:383–388.

14. Lichtenstein D, Mezière G, Biderman P, Gepner A. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000; 26:1434–1440.

15. Lichtenstein DA. Ultrasound in the management of thoracic disease. Crit Care Med. 2007; 35:5 Suppl. S250–S261.

16. Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill: lung sliding. Chest. 1995; 108:1345–1348.

17. Lichtenstein DA, Mezière G, Lascols N, Biderman P, Courret JP, Gepner A, Goldstein I, Tenoudji-Cohen M. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005; 33:1231–1238.

18. Mathis G, Bitschnau R, Gehmacher O, Scheier M, Kopf A, Schwärzler B, Amann T, Doringer W, Hergan K. Chest ultrasound in diagnosis of pulmonary embolism in comparison to helical CT. Ultraschall Med. 1999; 20:54–59.

19. Piette E, Daoust R, Denault A. Basic concepts in the use of thoracic and lung ultrasound. Curr Opin Anaesthesiol. 2013; 26:20–30.

20. Reissig A, Heyne JP, Kroegel C. Sonography of lung and pleura in pulmonary embolism: sonomorphologic characterization and comparison with spiral CT scanning. Chest. 2001; 120:1977–1983.

21. Reißig A, Kroegel C. Accuracy of transthoracic sonography in excluding post-interventional pneumothorax and hydropneumothorax. Comparison to chest radiography. Eur J Radiol. 2005; 53:463–470.

22. Reissig A, Kroegel C. Transthoracic ultrasound of lung and pleura in the diagnosis of pulmonary embolism: a novel non-invasive bedside approach. Respiration. 2003; 70:441–452.

23. Sistrom CL, Reiheld CT, Gay SB, Wallace KK. Detection and estimation of the volume of pneumothorax using real-time sonography: efficacy determined by receiver operating characteristic analysis. AJR Am J Roentgenol. 1996; 166:317–321.

24. Soldati G, Testa A, Pignataro G, Portale G, Biasucci DG, Leone A, Silveri NG. The ultrasonographic deep sulcus sign in traumatic pneumothorax. Ultrasound Med Biol. 2006; 32:1157–1163.

25. Soldati G, Testa A, Sher S, Pignataro G, La Sala M, Silveri NG. Occult traumatic pneumothorax: diagnostic accuracy of lung ultrasonography in the emergency department. Chest. 2008; 133:204–211.
26. Targhetta R, Bourgeois JM, Chavagneux R, Balmes P. Diagnosis of pneumothorax by ultrasound immediately after ultrasonically guided aspiration biopsy. Chest. 1992; 101:855–856.

27. Targhetta R, Bourgeois JM, Chavagneux R, Coste E, Amy D, Balmes P, Pourcelot L. Ultrasonic signs of pneumothorax: preliminary work. J Clin Ultrasound. 1993; 21:245–250.

28. Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T. International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012; 38:577–591.

29. Warner BW, Bailey WW, Shipley RT. Value of computed tomography of the lung in the management of primary spontaneous pneumothorax. Am J Surg. 1991; 162:39–42.

31. Wernecke K, Galanski M, Peters PE, Hansen J. Pneumothorax: evaluation by ultrasound--preliminary results. J Thorac Imaging. 1987; 2:76–78.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download