Abstract
Olfactory impairment is the most common clinical manifestation among the elderly, and its prevalence increases sharply with age. Notably, growing evidence has shown that olfactory dysfunction is the first sign of neurodegeneration, indicating the importance of olfactory assessment as an early marker in the diagnosis of neurological disorders. In this review, we describe the nature of olfactory dysfunction and the advantage of using animal models in olfaction study, and we include a brief introduction to olfactory behavior tests widely used in this field. The contribution of microglia in the neurodegenerative processes including olfactory impairment is then discussed to provide a comprehensive description of the physiopathological role of interactions between neurons and microglia within the olfactory system.
Olfaction is essential for survival and enhances quality of life as it is indispensable for the acquisition of various emotions, episodic memories, and social behaviors [3156]. A variety of specialized neurons responsible for olfactory information processing are localized in the olfactory epithelium (OE), the olfactory bulb (OB), and in other higher order olfactory centers such as the piriform cortex and the orbitofrontal cortex [68]. Anatomical or molecular disturbances in this system can lead to olfactory impairment, either as conductive or sensorineural loss. Conductive impairment is caused by mechanical obstruction of the olfactory apparatus preventing the delivery of odor stimulant to the OE, while the latter is associated with abnormal processing of stimuli in the OE and central olfactory system due to a defect in the neuronal network [2446]. Olfactory dysfunction is a very common clinical condition with a high prevalence; Lee et al. [66] reported that 4.5% of adults (range, 20–95 years) have self-awareness of olfactory dysfunction in Korea. The clinical implications of olfaction have been often unnoticed or underestimated, and recovery from olfactory loss remains challenging with limited therapeutic options, especially in the case of sensorineural olfactory defects [87].
Although sinonasal disease and upper respiratory infection, as well as head trauma, are the most commonly reported clinical manifestations leading to transient or persistent olfactory disturbance [244656], the aging process has been regarded as a major contributing factor for anosmia in general [86186]. It has been reported that more than half of ‘healthy’ individuals over age 65 have a decline in olfaction, and the number of victims increases steeply with aging to nearly 80% in those over 80 years of age [41]. In addition, patients with a variety of neurological diseases including Alzheimer's disease (AD), Huntington's disease (HD), Parkinson's disease (PD), multiple sclerosis, and dementia show a high susceptibility to olfactory dysfunction [948]. Interestingly, olfactory loss seems to precede the onset of other typical neurodegenerative symptoms such as memory loss and motor impairment, indicating the importance of olfactory assessment for an early diagnosis of various neurological disorders [7885101]. Therefore, olfactory disorders have become a matter of societal as well as individual importance in an era of accelerated population aging with concurrent increasing risks of neuropathological medical conditions [1834]. Moreover, elucidating the underlying mechanisms of olfactory loss might be necessary not only to understand the nature of neurodegeneration but also to develop disease management strategies for treatment and prevention.
Age-related physiological changes such as an increased susceptibility to infection, long-term exposure of air-borne agents (e.g., smoking, air pollutant), and blood flow reduction in the nasal mucosa can cause direct damage to olfactory neurons [4088]. Autopsy and brain imaging have revealed gradual atrophy of the OB accompanied by the loss of olfactory nerve fibers with aging [1725]. Similarly, functional MRI and electroencephalography recordings have demonstrated a neurodegeneration-associated decline in olfactory responsiveness with decreased neural activity in patients [1327286999]. Some researchers have suggested a correlation between the development of typical neuropathological signs such as protein aggregates and the severity of olfactory dysfunction; post-mortem brain studies have shown that amyloid beta plaque and neurofibrillary tangles were often accumulated in the OB neuronal dendrites in AD patients [57102], and intracytoplasmic Lewy bodies induced by α-synuclein aggregation were identified in the OE and OB neurons of PD patients [2135], while others found no significant correlations [10]. In addition, defects in neurotransmitter signaling seem to impede proper neural interactions for olfactory processing in a context-dependent manner. In particular, acetylcholine might play a critical role in maintaining normal olfaction in humans [1419]; indeed, Bohnen et al. [19] reported that enhanced cholinesterase activity not only decreased acetylcholine concentration but also led to olfactory decline. On the other hand, donepezil, a cholinesterase inhibitor, can restore the impaired olfaction of AD patients [12,97]. Interestingly, despite dopamine deficiency being regarded as a key pathophysiologic feature of PD, neither dopaminergic neuronal denervation in the OB nor treatment with dopaminergic agents has improved olfaction of PD patients, indicating the complicated nature of region-specific neurodegenerative processes [319]. Therefore, assessment of the brain via indirect imaging or post-mortem studies would be insufficient in explaining in detail how olfactory dysfunction begins and develops during aging and neurodegeneration. In this regard, rodent models have significantly contributed to the establishment and evaluation of hypotheses in these fields.
Animal models are indispensable in the investigation of disease pathogenesis and the development of relevant therapeutics. Rodent models are the most preferred experimental species due to their genomic similarity to humans, convenience for genetic modification, and cost-effectiveness. Rodent models can recapitulate the neurological molecular pathology including the production of protein aggregates and alterations in the neurotransmitter system. Moreover, they have been widely used to distinguish between cause and effect and to determine the clinical significance of novel findings by modulating (either up- or down-regulation) specific signalings in vivo.
Olfaction is gradually impaired with age or neurodegeneration in rodents, and, in both rodent and human, the cellular mechanisms of olfactory dysfunction have been studied. It has been reported that olfactory loss during aging is associated with a reduction in the number and diversity of olfactory neurons caused by: i) cell cycle arrest in the OE neuroblast; ii) significant decline in neurogenesis in the subventricular zone (SVZ); or iii) transcriptional down-regulation in olfactory receptor subtypes [657089]. In neurological disease models, toxic metabolites and protein aggregates with secondary neuroinflammatory molecules seem to accelerate olfactory deficit (details presented in the following). Therefore, rodent models can provide valuable information regarding cellular and molecular mechanisms associated with olfactory impairment for application in establishing therapeutic strategies.
To date, various psychophysical, electrophysiological, and psychophysiological tests for olfactory function have been developed to quantify and qualify olfaction in the clinic [1339]. Because of its relatively simple and intuitive procedure, psychophysical olfactory testing has been widely used, with some modifications, to evaluate the olfaction of experimental animals. Odor recognition, identification, and discrimination abilities are the key criteria for olfactory assessment using psychophysical olfactory tests.
In this review, we briefly introduce the characteristics and principle procedures of the most frequently used olfactory tests for experimental mice (for more detail, refer to [1538103107]). Note that in these tests, each subject should perform the task once to avoid errors derived from unintended behavioral learning rather than assessing pure olfaction, except in the case of testing long-term olfactory memory formation ability.
In these tests, animals are simultaneously exposed to unfamiliar, novel odor cues and control odor cues such as water. During testing, various concentrations of the odors are introduced to subjects in a descending order (from high to low concentration) and the total exploring times between stimulus and water cues are compared to determine ‘olfactory sensitivity’. In general, subjects with normal olfactory function are expected to spend more time investigating the higher stimulus concentration than the lower concentration of the stimulus or the water control.
The basic test format can be modified for use in advanced testing such as testing olfactory preference and olfactory avoidance. These tests are aimed at assessing the ability to distinguish between attractive (e.g., vanilla or peanut butter scent are preferred) or aversive scents (e.g., 2-methylbutyric acid) with the results based on total exploratory times.
These tests are useful in determining whether mice can discriminate a novel odor stimulus from familiar ones. During the olfactory habituation-dishabituation test, which is considered a basic olfactory discrimination test, subjects are first presented with odor ‘A’ for habituation. Subject response to odor A decreases with repeated exposure to the odor (i.e., habituated behavior). When a different odor, ‘B’, is introduced to mice during this test procedure, exploring time to the novel stimulus will be increased (i.e., dishabituated behavior).
With some modification, this test can be applied as a ‘social recognition test’ to determine whether mice can discriminate ‘self’ and ‘non-self’ by using olfaction (note that the original olfactory discrimination test is also known as ‘non-social recognition test’ in contrast to the social recognition test). In the social recognition test, the subject is exposed to blocks from its own cage and from another non-self cage and exploration time for each block is quantified. A standard wild-type mouse always tends to show a preferential interest in the block from the non-self, foreign animal.
Finally, habituation-dishabituation behavior is also used for the assessment of olfactory memory by evaluating the ‘rewarded olfactory discrimination’ ability of subjects, although this test is usually used to evaluate cognitive function rather than olfaction itself.
The buried food test is one of the most frequently used tests for olfactory evaluation and is useful in determining an animal's olfactory perceptibility. Prior to the test, animals undergo food restriction to increase food motivation behavior. Then the subject is introduced into the test cage with food buried under the bedding, and the latent period to locate and eat the food is recorded. Control testing with ‘exposed’ food (i.e., located on the bedding) should be performed in parallel with the buried food test to confirm that subject is free from any appetite disorder or visual defect. Typically, mice with olfactory deficit show an increased latency in the buried food test but not in the exposed food test.
The central nervous system (CNS) consists of neurons and supportive glial cells including oligodendrocytes, astrocytes, and microglia. Unlike other CNS cells that are derived from an ectodermal lineage, microglia are known to originate from the mesodermal hematopoietic lineage, and their primitive progenitors in the yolk sac migrate into the developing brain during early embryogenesis [47]. Microglia perform a constant surveillance activity, thereby being considered as the resident macrophages in the CNS, and they maintain their population via consistent local proliferation throughout the lifetime of the CNS [1].
In the normal mature CNS, the majority of microglia are under a steady state with ramified morphology; so-called ‘resting microglia.’ In addition to their immune surveillance role, resting microglia contribute to many biological processes in the CNS including neurogenesis [93]. It has been previously reported that microglia participate in the arrangement of appropriate neural circuits by removing cell debris during the embryonic period and early postnatal life [6], and, even in the resting state, microglia residing near a neurogenic center such as the OB and subgranular zone are reported to perform phagocytosis of apoptotic cells during neurogenesis [8493]. When microglia are activated in response to various environmental stimuli, they expand their population (microgliosis) as well as transform into an amoeboid-like morphology with increased secretion of cytokines and inflammatory mediators. Indeed, almost every neuropathic condition is accompanied by extensive microglial activation and profound microgliosis [5775106]. Activatation of microglia is often divided into two states, classical activation (M1) and alternative activation (M2), based on the activating factors (M1: lipopolysaccharide [LPS] and interferon γ [IFN-γ]; M2: interleukin [IL]-4 and IL-13), expressed cell specific markers (M1: CD86; M2: CD206), and the main biological functions (M1: production of proinflammatory molecules; M2: enhanced phagocytosis); however, there are some limitations in this M1-M2 microglial classification system that mainly stem from the fact that the system overlooks the plasticity and complexity of microglia and because there is no actual boundary between the two phenotypes [71]. Microglia can provide both beneficial and detrimental effects on neurodegenerative process depending on the context; however, it is generally accepted that M1 microglia aggravate an on-going neuropathology by producing neurotoxic factors, while M2 microglia help to resolve the problem via phagocytosis of unwanted molecules. For instance, classically activated microglia-derived proinflammatory cytokines such as IL-1β and tumor necrosis factor α (TNF-α) reduce the number of newborn cells in neurogenic centers and impede normal synaptic activity in neurons, as is frequently observed in aging and neurodegenerative disorders [76]. High levels of nitric oxide and reactive oxygen species also contribute to microglia-induced neural apoptosis [3367]. The M1 microglia-derived proinflammatory signals also stimulate neighboring astrocytes to proliferate and produce cytotoxic factors, resulting in more severe neuroinflammation [98]. In contrast, several studies have shown that phagocytotic ability of alternatively activated microglia is important to prevent/delay the development of neurodegeneration by removing the causative agents in several neurologic disorders (e.g., amyloid beta in AD) and restricting the affected lesions [5860]. Therefore, comprehensive understanding of microglial roles in various neuropathologic conditions should precede testing of the therapeutic potential of candidate drugs that modify microglial activity.
One of the main neurogenic centers, the SVZ, possesses adult neural stem cells and produces neural progenitors upon stimulation. These neuroblasts traverse the rostral migratory stream to the OB, where they mature and differentiate into granule cells and periglomerular neurons [22]. Although there is debate on the presence of OB neurogenesis in human adults, it has been reported that newly generated OB neurons seem to be essential for the maintenance of normal olfaction in animals because a chronic treatment of the antimitotic agent AraC ablated SVZ-derived neurogenesis and was followed by olfactory deficits in mice [22].
It has been previously reported that microglia participate in the arrangement of the appropriate neural circuits during neurogenesis by removing cell debris during the embryonic period and postnatal life [684]. As part of the SVZ neurogenic center, therefore, the OB contains a high number of microglia, which allows investigation of the role of microglia in neural differentiation, survival, and apoptosis as well as microglia-neuronal interaction [80]. The number of phagocytic amoeboid-like microglia in the rat OB gradually increases during the first 3 to 4 weeks after birth then decreases as structural and functional stabilization of OB circuitry becomes established [2642]. Recently, Ribeiro Xavier et al. [77] reported a distinctive subtype of microglia within the SVZ-OB axis. These microglia had an amoeboid-like morphology with an enlarged cell body and produced M2-like cytokines such as IL-4 and IL-10. It has also been noted that microglial depletion via intravenous injection of Cd11b-conjugated saporin toxin resulted in a decreased number of OB neuroblasts migrating from the SVZ in rodents, indicating that appropriate microglial activation might be essential for the survival and migration of neuroblasts in the SVZ-OB axis [77].
In addition, OB neural damage is often accompanied by neuroinflammation in response to pathologic stimuli. In general, microgliosis and astrocytosis develop simultaneously during the inflammatory process. Some studies have shown the morphological diversity of astrocyte subtypes and their expression of synaptogenetic molecules in the OB layers, implying that astrocytes can also contribute to OB structure formation during the developmental stage [111245]; however, direct evidence of astrocytic involvement in olfactory loss and their possible roles in OB pathogenesis has not been reported yet. On the other hand, the correlation between microglial activity and the development of olfactory dysfunction in various pathologic conditions has been discussed, as summarized in the following.
It has been reported that occluding one naris during the early postnatal period significantly alters olfactory neuronal connectivity, leading to an olfactory deficit [23]. Histologically, unilateral naris closure has resulted in a decline in OB size due to massive loss of tyrosine hydroxylase-positive dopaminergic interneurons in the OB [50]. Notably, progressive microgliosis within the OB was also observed with the morphological transformation from resting state into the amoeboid state, indicating increased phagocytic activity. As a result, most adult-born OB neurons are engulfed by microglia in the occluded OB, leading to the loss of synaptic activity and olfactory dysfunction [3650].
Dichlobenil administration is another widely used method for OB deafferentation as it leads to a rapid degeneration of olfactory sensory neurons (OSNs) by disrupting their axonal projections [63105]. Importantly, it is reported that dichlobenil-induced ablation of OE neurons not only decreased the survival of newly generated neurons in the OB but also increased the proliferation of microglia (but not of astrocytes) similar to the results obtained from a unilateral naris closure model [63]. To determine the cause and effect between olfactory loss and microgliosis, the anti-inflammatory agent minocycline was administrated to dichlobenil-treated mice, then, olfactory behavioral tests were conducted. It was reported that minocycline treatment could prevent neuronal apoptosis and olfactory impairment, indicating that suppression of microglial activity is protective of olfactory deafferentation and functional deficits after olfactory ablation [63].
A prolonged inflammatory status such as chronic rhinosinusitis is a common predisposition to olfactory loss in patients. A transgenic mouse model of inducible olfactory inflammation under controlled expression of the microglia-derived proinflammatory cytokine TNF-α successfully showed that TNF-α can produce infiltration of inflammatory cells into the OE [62]. It is also reported that neural synaptic activity, as determined by electro-olfactogram (EOG) recordings, was altered in a TNF-α concentration-dependent manner; indeed, the EOG response was almost abrogated upon long-term induction of TNF-α, while it recovered to a normal level after TNF-α withdrawal. Moreover, TNF-α can suppress regenerative capacity in the OE by inhibiting the proliferation of neural progenitors and impeding the basal turn-over rate of mature olfactory neurons [62].
The contribution of microglia-derived proinflammatory cytokines in the olfactory system has been also described in bacterial infection models [5294]. In these studies, mice were challenged with Staphylococcus aureus after Triton X-100 (Sigma-Aldrich, USA) treatment in the ipsilateral nasalis. Infection of S. aureus led to a significant olfactory damage accompanied by rapid increases in proinflammatory cytokines IL-6 and TNF-α. In addition, inducible nitric oxide synthase-expressing microglia were accumulated in the OE and OB in response to bacterial exposure [52]. Although the physiological meaning of microgliosis following bacterial infection has not been fully described, microglia can play key roles in the development and resolution of inflammatory responses in the olfactory system [87].
Using simple but reliable behavior tests reveals that rodents bearing neurodegenerative conditions usually show the distinctive signs of olfactory loss similar to those of humans (Table 1). AD-affected transgenic mice are the most widely studied animal model for olfactory loss, and most related papers have focused on olfactory memory loss or learning ability defects because AD is mainly associated with defective hippocampal cognitive CNS functions in humans [95108110]. In the OB, neuronal death and neurite abnormality have been observed with cholinergic- and noradrenergic signaling defects, leading to olfactory dysfunction in AD mice [51]. It has been also noted that amyloid beta deposits were found in the AD-affected OB early in life and accumulated throughout the olfactory system with age [100]. Defective neuronal diversity with decreased neurogenic activity in the OB has been also reported in other neurodegenerative disease mice as summarized in Table 1. These findings have indicated the contribution of neuronal abnormality to the development of olfactory loss in neurodegenerative diseases, although little has been reported about the molecular mechanism(s) underlying neural defects in the olfactory system.
In general, microgliosis is a common pathological feature of neurodegeneration throughout the brain, and abnormal microglial activation in the OB is clearly observed in AD- and PD-affected patients [37]. However, contribution of microglia to olfactory defects has been somewhat underestimated because microgliosis is often regarded as an inevitable outcome of autonomous neural damage. In this regard, we have studied the correlation between olfaction and microgliosis by using mice models for Niemann-Pick disease type C (NPC), a rare but fatal neurodegenerative disorder, to elucidate the neuropathological effects of microglial activity on olfactory loss during the neurodegenerative process.
NPC is an autosomal recessive, incurable disease found in approximately one among 120,000 live births [96]. Ninety-five percent of NPC cases result from genetic mutations in the NPC1 gene, referred to as NPC type C1 (NPC1), while the remaining 5% of cases originate from the NPC2 gene mutation, referred to as NPC type C2 (NPC2) [74]. Both NPC1 and NPC2 proteins are associated with the process of cholesterol egress in late endosomes and lysosomes, although the exact mechanism of NPC1/2-mediated cholesterol trafficking has not been fully elucidated [90]. Because NPC1/2 proteins are ubiquitously expressed in mammalian cells, NPC is considered a neurovisceral disorder, and its pathologic signs are easily observed in various organs including liver, spleen, and lung, but the CNS is the most affected region [9096]. Importantly, the severity of neurological symptoms is regarded as the most decisive factor for the determining the prognosis of NPC because neurodegeneration is ultimately responsible for the death of most patients. Indeed, delayed development of neurological symptoms seems to be correlated with longer survival in patients [4453]. Therefore, therapeutic strategies for NPC management are mainly focused on the early detection, prevention, and amelioration of neuropathologic signs of patients [73].
To date, noticeable sign of an olfactory defect in NPC patients have not been reported; however, growing evidence collected from animal studies has suggested the presence of olfactory dysfunction in NPC, as has been observed in other neurological disorders. Hovakimyan et al. [54] reported that electrophysiological activity of olfactory mucosa derived from the OE was significantly decreased in response to various odor stimuli in NPC mice compared to the activity level in the wild-type counterpart. More direct evidence of olfactory dysfunction in NPC mice was obtained from the results of buried food finding tests. During those tests, normal mice successfully located the hidden food within the prescribed time limit (3 min) while NPC mice had difficulty in carrying out the task, showing a high failure rate, thus implying that olfaction is impaired in NPC status mice [83]. Histologically, filipin staining revealed massive accumulations of cholesterol in the principal components of the olfactory system, the OE and OB (Fig. 1). Furthermore, the numbers of mature OSNs and periglomerular neurons were profoundly decreased in these regions, partially due to the reduced survival of precursors for OSNs and OB neuroblasts (Fig. 2) [83], supporting the suggestion that axodendritic synaptic activity between OE and OB might be insufficient for normal processing of olfactory information in NPC mice. Interestingly, abnormal microglial activation was observed in the NPC-affected OB, and the anti-inflammatory agent cyclosporin A (CsA) successfully prevented abnormal microgliosis and immature neuronal apoptosis in the NPC-affected OB, leading to the recovery of olfaction [83].
Activated microglia can perform multiple functions including cytokine production, phagocytosis, and antigen presentation by interacting with neighboring neurons via the production of several soluble factors to create coordinated neuroinflammatory or neuroprotective responses depending on the context [2949]. Therefore, we extended our previous studies by focusing on microglia-neuronal crosstalk to elucidate the underlying mechanisms of the massive microgliosis in the NPC-OB. It was noted that the olfactory deficit in NPC mice seems to be, in part, the result of bi-directional microglia-neuron interaction [82]. In detail, a higher level of neuronal chemokine Cx3cl1, which mediates neuronal-microglial communication through binding to its specific receptor CX3CR1 on microglia, was observed in NPC mice-derived cerebrospinal fluid (NPC-CSF) than in wild-type mice. Moreover, most Iba1-positive amoeboid NPC1 microglia expressed the Cx3cr1 marker, implying a correlation between microglial activation and Cx3cl1-Cx3cr1 signaling. To determine the biological and pathological significance of our results, we neutralized Cx3cl1 function with a specific Cx3cl1 antibody and then undertook histological analysis and behavioral tests. Importantly, blockage of Cx3cl1-Cx3cr1 interaction prevented neurotoxic microglial behavior and was followed by increased survival of OB neurons and partial recovery of olfactory loss in NPC1 mice. Moreover, upregulation of Cx3cl1-Cx3cr1 signaling was partially mediated by the catalytic activity of microglial cathepsin S (Ctss) because the nasal infusion of the Ctss inhibitor LHVS could successfully suppress microglial activation by reducing Cx3cl1 secretion into NPC-CSF. As a result, LHVS not only prevented OB neuronal damage, but it also improved the olfactory function of NPC1 mice. Finally, we suggested a causal link between NPC1 dysfunction and abnormal Ctss activity of NPC1 microglia; in detail, disturbed cholesterol homeostasis caused by NPC1 mutation led to cholesterol accumulation in the lysosome and, in turn, activated p38 MAPK signaling, resulting in Ctss activation and maturation (Fig. 3) [82]. Interestingly, the transcription level of Ctss was reported to be increased in the liver and cerebellum of NPC-affected patients [2], implying that the results from the mouse experiment could be applicable in clinical fields.
In this review, we have pointed out the possible contribution of microglia to olfactory impairment and, based on our recent studies with NPC mice, emphasized the usefulness of animal models in this field. In NPC status, the integrity of the central olfactory system is disrupted due to a cholesterol accumulation, causing excessive crosstalk between neurons and microglia via the Cx3cl1-Cx3cr1 axis and leading to olfactory dysfunction. Moreover, the application of specific inhibitors for the target molecule indicates therapeutic potential for olfactory recovery in NPC mice. Therefore, animal study can provide not only a thorough insight into understanding the nature of olfactory system, but also, it can inspire the development of novel therapeutic strategies for olfactory loss in humans.
Figures and Tables
Fig. 1
Cholesterol accumulation in the olfactory system of Niemann-Pick disease type C (NPC) mice. Representative filipin-stained images showing intracellular cholesterol in the olfactory epithelium (OE; A) and the olfactory bulb (OB; B) of 8-week-old wild-type (WT) and NPC model mice. Scale bars = 100µm (A and B).
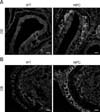
Fig. 2
Distribution patterns of olfactory sensory neurons and other periglomerular neurons in the olfactory epithelium (OE; A) and olfactory bulb (OB; B) revealed by immunohistochemical analysis. Relative expression intensity of olfactory marker protein (OMP) in Niemann-Pick disease type C (NPC) mice is down-regulated compared to that in wild-type (WT) controls in both the OE (A) and OB (B), indicating that the number of olfactory sensory neurons are decreased in NPC status. (C) Tyrosine hydroxylase (TH)-expressing neurons, a type of periglomerular neuron, are damaged in the OB of NPC mice compared to those of WT counterparts. Scale bars = 50 µm (A and C), 100 µm (B).
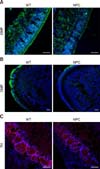
Fig. 3
A simplified schematic diagram of neuron-microglia involvement in olfactory dysfunction in Niemann-Pick disease type C (NPC) mice. Briefly, NPC mutation upregulates intracellular cholesterol followed by p38 MAPK signaling activation in microglia. In response to this activation, multiple cytotoxic molecules are secreted, leading to direct neurotoxicity. In addition, p38 MAPK signaling enhances the maturation of microglial cathepsin S, thereby increasing the shedding of neuronal chemokine Cx3cl1. The Cx3cl1 secretion results in the recruitment of Cx3cr1 (a specific receptor for Cx3cl1)-bearing microglia, which eventually results in repetitive cycling between neurons and microglia in the NPC-affected OB. Importantly, blockage of this pathway either by a Cx3cl1 neutralizing antibody or by the cathepsin S inhibitor LHVS successfully prevents microglial activation and, in turn, restores olfaction of NPC mice. This illustration is a modified version of the ‘The Table of Contents Image’ in our recently published article as stated. Source: Seo Y, et al. Glia 2016, 64(12), 2291-2305.
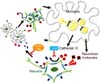
Table 1
Olfactory dysfunction in neurodegenerative mice models

AD, Alzheimer's disease; PD, Parkinson's disease; HD, Huntington's disease; NPC, Niemann-Pick disease type C; OB, olfactory bulb; +, evidence of symptom; −, no evidence of symptom; PSA-NCAM, polysialylated-neural cell adhesion molecule; NA, not available; OE, olfactory epithelium; NP, not performed; TH, tyrosine hydroxylase; SVZ, subventricular zone.
Acknowledgments
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant HI14C1443), by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (No. 2012M3A9C6049716), partially by the Research Institute for Veterinary Science, Seoul National University (Republic of Korea), and partially supported by a clinical research grant from Pusan National University Hospital in 2017.
References
1. Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007; 10:1538–1543.

2. Alam MS, Getz M, Yi S, Kurkewich J, Safeukui I, Haldar K. Plasma signature of neurological disease in the monogenetic disorder Niemann-Pick Type C. J Biol Chem. 2014; 289:8051–8066.

3. Albers MW, Tabert MH, Devanand DP. Olfactory dysfunction as a predictor of neurodegenerative disease. Curr Neurol Neurosci Rep. 2006; 6:379–386.

4. Alvarado-Martínez R, Salgado-Puga K, Peña-Ortega F. Amyloid beta inhibits olfactory bulb activity and the ability to smell. PLoS One. 2013; 8:e75745.

5. Arnold SE, Lee EB, Moberg PJ, Stutzbach L, Kazi H, Han LY, Lee VM, Trojanowski JQ. Olfactory epithelium amyloid-beta and paired helical filament-tau pathology in Alzheimer disease. Ann Neurol. 2010; 67:462–469.

6. Ashwell K. The distribution of microglia and cell death in the fetal rat forebrain. Brain Res Dev Brain Res. 1991; 58:1–12.

7. Attems J, Jellinger KA. Olfactory tau pathology in Alzheimer disease and mild cognitive impairment. Clin Neuropathol. 2006; 25:265–271.
8. Attems J, Walker L, Jellinger KA. Olfaction and aging: a mini-review. Gerontology. 2015; 61:485–490.

9. Attems J, Walker L, Jellinger KA. Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol. 2014; 127:459–475.

10. Bahar-Fuchs A, Chételat G, Villemagne VL, Moss S, Pike K, Masters CL, Rowe C, Savage G. Olfactory deficits and amyloid-β burden in Alzheimer's disease, mild cognitive impairment, and healthy aging: a PiB PET study. J Alzheimers Dis. 2010; 22:1081–1087.

11. Bailey MS, Puche AC, Shipley MT. Development of the olfactory bulb: evidence for glia-neuron interactions in glomerular formation. J Comp Neurol. 1999; 415:423–448.

12. Bailey MS, Shipley MT. Astrocyte subtypes in the rat olfactory bulb: morphological heterogeneity and differential laminar distribution. J Comp Neurol. 1993; 328:501–526.

13. Barresi M, Ciurleo R, Giacoppo S, Foti Cuzzola V, Celi D, Bramanti P, Marino S. Evaluation of olfactory dysfunction in neurodegenerative diseases. J Neurol Sci. 2012; 323:16–24.

14. Beach TG, Kuo YM, Spiegel K, Emmerling MR, Sue LI, Kokjohn K, Roher AE. The cholinergic deficit coincides with Abeta deposition at the earliest histopathologic stages of Alzheimer disease. J Neuropathol Exp Neurol. 2000; 59:308–313.

15. Berkley MA, Stebbins WC. Comparative Perception. New York: Wiley;1990.
16. Bernal-Mondragón C, Rivas-Arancibia S, Kendrick KM, Guevara-Guzmán R. Estradiol prevents olfactory dysfunction induced by A-β 25-35 injection in hippocampus. BMC Neurosci. 2013; 14:104.

17. Bhatnagar KP, Kennedy RC, Baron G, Greenberg RA. Number of mitral cells and the bulb volume in the aging human olfactory bulb: a quantitative morphological study. Anat Rec. 1987; 218:73–87.

18. Boesveldt S, Lindau ST, McClintock MK, Hummel T, Lundstrom JN. Gustatory and olfactory dysfunction in older adults: a national probability study. Rhinology. 2011; 49:324–330.

19. Bohnen NI, Müller ML, Kotagal V, Koeppe RA, Kilbourn MA, Albin RL, Frey KA. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson's disease. Brain. 2010; 133:1747–1754.

20. Bonito-Oliva A, Masini D, Fisone G. A mouse model of non-motor symptoms in Parkinson's disease: focus on pharmacological interventions targeting affective dysfunctions. Front Behav Neurosci. 2014; 8:290.

21. Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rüb U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson's disease (preclinical and clinical stages). J Neurol. 2002; 249:Suppl 3. III/1–III/5.

22. Breton-Provencher V, Lemasson M, Peralta MR 3rd, Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J Neurosci. 2009; 29:15245–15257.

23. Brunjes PC. Unilateral naris closure and olfactory system development. Brain Res Brain Res Rev. 1994; 19:146–160.

24. Burkert S, Haberland EJ, Gudziol H. [Olfactory and gustatory disorders–causes, diagnosis and treatment]. MMW Fortschr Med. 2005; 147:4951–53. German.
25. Buschhüter D, Smitka M, Puschmann S, Gerber JC, Witt M, Abolmaali ND, Hummel T. Correlation between olfactory bulb volume and olfactory function. Neuroimage. 2008; 42:498–502.

26. Caggiano AO, Brunjes PC. Microglia and the developing olfactory bulb. Neuroscience. 1993; 52:717–724.

27. Caminiti F, Ciurleo R, Bramanti P, Marino S. Persistent anosmia in a traumatic brain injury patient: role of orbitofrontal cortex. Brain Inj. 2013; 27:1715–1718.

28. Caminiti F, De Salvo S, De Cola MC, Russo M, Bramanti P, Marino S, Ciurleo R. Detection of olfactory dysfunction using olfactory event related potentials in young patients with multiple sclerosis. PLoS One. 2014; 9:e103151.

29. Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005; 48:16–42.

30. Cassano T, Romano A, Macheda T, Colangeli R, Cimmino CS, Petrella A, LaFerla FM, Cuomo V, Gaetani S. Olfactory memory is impaired in a triple transgenic model of Alzheimer disease. Behav Brain Res. 2011; 224:408–412.

31. Cheal M. Social olfaction: a review of the ontogeny of olfactory influences on vertebrate behavior. Behav Biol. 1975; 15:1–25.

32. Cheng D, Logge W, Low JK, Garner B, Karl T. Novel behavioural characteristics of the APPSwe/PS1ΔE9 transgenic mouse model of Alzheimer's disease. Behav Brain Res. 2013; 245:120–127.

33. Colton CA, Gilbert DL. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett. 1987; 223:284–288.

34. Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life--an updated review. Chem Senses. 2014; 39:185–194.

35. Daniel SE, Hawkes CH. Preliminary diagnosis of Parkinson's disease by olfactory bulb pathology. Lancet. 1992; 340:186.

36. Denizet M, Cotter L, Lledo PM, Lazarini F. Sensory deprivation increases phagocytosis of adult-born neurons by activated microglia in the olfactory bulb. Brain Behav Immun. 2017; 60:38–43.

37. Doorn KJ, Goudriaan A, Blits-Huizinga C, Bol JG, Rozemuller AJ, Hoogland PV, Lucassen PJ, Drukarch B, van de Berg WD, van Dam AM. Increased amoeboid microglial density in the olfactory bulb of Parkinson's and Alzheimer's patients. Brain Pathol. 2014; 24:152–165.

38. Doty RL. Handbook of Olfaction and Gustation. 3rd ed. Hoboken: Wiley;2015.
39. Doty RL. Olfactory dysfunction and its measurement in the clinic and workplace. Int Arch Occup Environ Health. 2006; 79:268–282.

41. Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984; 226:1441–1443.

42. Fiske BK, Brunjes PC. Microglial activation in the developing rat olfactory bulb. Neuroscience. 2000; 96:807–815.

43. Fleming SM, Tetreault NA, Mulligan CK, Hutson CB, Masliah E, Chesselet MF. Olfactory deficits in mice overexpressing human wildtype alpha-synuclein. Eur J Neurosci. 2008; 28:247–256.
44. Fraile PQ, Hernández EM, Martínez de Aragón A, Macias-Vidal J, Coll MJ, Espert AN, Silva MT. [Niemann-Pick type C disease: from neonatal cholestasis to neurological degeneration. Different phenotypes]. An Pediatr. 2010; 73:257–263. Spanish.
45. García-Marqués J, López-Mascaraque L. Clonal mapping of astrocytes in the olfactory bulb and rostral migratory stream. Cereb Cortex. 2017; 27:2195–2209.

46. Getchell TV. Smell and Taste in Health and Disease. New York: Raven Press;1991.
47. Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Front Cell Neurosci. 2013; 7:45.

48. Godoy MD, Voegels RL, Pinna Fde R, Imamura R, Farfel JM. Olfaction in neurologic and neurodegenerative diseases: a literature review. Int Arch Otorhinolaryngol. 2015; 19:176–179.

49. Gomes FC, Spohr TC, Martinez R, Moura Neto V. Cross-talk between neurons and glia: highlights on soluble factors. Braz J Med Biol Res. 2001; 34:611–620.

50. Grier BD, Belluscio L, Cheetham CE. Olfactory sensory activity modulates microglial-neuronal interactions during dopaminergic cell loss in the olfactory bulb. Front Cell Neurosci. 2016; 10:178.

51. Guérin D, Sacquet J, Mandairon N, Jourdan F, Didier A. Early locus coeruleus degeneration and olfactory dysfunctions in Tg2576 mice. Neurobiol Aging. 2009; 30:272–283.

52. Herbert RP, Harris J, Chong KP, Chapman J, West AK, Chuah MI. Cytokines and olfactory bulb microglia in response to bacterial challenge in the compromised primary olfactory pathway. J Neuroinflammation. 2012; 9:109.

53. Héron B, Ogier H. [Niemann-Pick type C disease: clinical presentations in pediatric patients]. Arch Pediatr. 2010; 17:Suppl 2. S45–S49. French.
54. Hovakimyan M, Meyer A, Lukas J, Luo J, Gudziol V, Hummel T, Rolfs A, Wree A, Witt M. Olfactory deficits in Niemann-Pick type C1 (NPC1) disease. PLoS One. 2013; 8:e82216.

55. Kandasamy M, Rosskopf M, Wagner K, Klein B, Couillard-Despres S, Reitsamer HA, Stephan M, Nguyen HP, Riess O, Bogdahn U, Winkler J, von Horsten S, Aigner L. Reduction in subventricular zone-derived olfactory bulb neurogenesis in a rat model of Huntington's disease is accompanied by striatal invasion of neuroblasts. PLoS One. 2015; 10:e0116069.

56. Keller A, Malaspina D. Hidden consequences of olfactory dysfunction: a patient report series. BMC Ear Nose Throat Disord. 2013; 13:8.

57. Kingwell K. Neurodegenerative disease: microglia in early disease stages. Nat Rev Neurol. 2012; 8:475.
58. Kiyota T, Okuyama S, Swan RJ, Jacobsen MT, Gendelman HE, Ikezu T. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer's disease-like pathogenesis in APP+PS1 bigenic mice. FASEB J. 2010; 24:3093–3102.

59. Kohl Z, Regensburger M, Aigner R, Kandasamy M, Winner B, Aigner L, Winkler J. Impaired adult olfactory bulb neurogenesis in the R6/2 mouse model of Huntington's disease. BMC Neurosci. 2010; 11:114.

60. Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008; 216:15–24.

61. Lafreniere D, Mann N. Anosmia: loss of smell in the elderly. Otolaryngol Clin North Am. 2009; 42:123–131.

62. Lane AP, Turner J, May L, Reed R. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. J Neurosci. 2010; 30:2324–2329.

63. Lazarini F, Gabellec MM, Torquet N, Lledo PM. Early activation of microglia triggers long-lasting impairment of adult neurogenesis in the olfactory bulb. J Neurosci. 2012; 32:3652–3664.

64. Lazic SE, Goodman AO, Grote HE, Blakemore C, Morton AJ, Hannan AJ, van Dellen A, Barker RA. Olfactory abnormalities in Huntington's disease: decreased plasticity in the primary olfactory cortex of R6/1 transgenic mice and reduced olfactory discrimination in patients. Brain Res. 2007; 1151:219–226.

65. Lee AC, Tian H, Grosmaitre X, Ma M. Expression patterns of odorant receptors and response properties of olfactory sensory neurons in aged mice. Chem Senses. 2009; 34:695–703.

66. Lee WH, Wee JH, Kim DK, Rhee CS, Lee CH, Ahn S, Lee JH, Cho YS, Lee KH, Kim KS, Kim SW, Lee A, Kim JW. Prevalence of subjective olfactory dysfunction and its risk factors: Korean National Health and Nutrition Examination Survey. PLoS One. 2013; 8:e62725.

67. Liu B, Gao HM, Wang JY, Jeohn GH, Cooper CL, Hong JS. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann N Y Acad Sci. 2002; 962:318–331.

68. Lledo PM, Gheusi G, Vincent JD. Information processing in the mammalian olfactory system. Physiol Rev. 2005; 85:281–317.

69. Lötsch J, Hummel T. The clinical significance of electrophysiological measures of olfactory function. Behav Brain Res. 2006; 170:78–83.

70. Mirich JM, Williams NC, Berlau DJ, Brunjes PC. Comparative study of aging in the mouse olfactory bulb. J Comp Neurol. 2002; 454:361–372.

71. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008; 8:958–969.

72. Nathan BP, Yost J, Litherland MT, Struble RG, Switzer PV. Olfactory function in apoE knockout mice. Behav Brain Res. 2004; 150:1–7.

73. Ogier de. [Niemann-Pick type C disease: an early diagnosis for a therapeutic hope]. Arch Pediatr. 2010; 17:S39–S40. French.
74. Pentchev PG, Comly ME, Kruth HS, Vanier MT, Wenger DA, Patel S, Brady RO. A defect in cholesterol esterification in Niemann-Pick disease (type C) patients. Proc Natl Acad Sci U S A. 1985; 82:8247–8251.

75. Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010; 6:193–201.

76. Pickering M, Cumiskey D, O'Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol. 2005; 90:663–670.

77. Ribeiro Xavier AL, Kress BT, Goldman SA, Lacerda de, Nedergaard M. A distinct population of microglia supports adult neurogenesis in the subventricular zone. J Neurosci. 2015; 35:11848–11861.

78. Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, Launer L, White LR. Association of olfactory dysfunction with risk for future Parkinson's disease. Ann Neurol. 2008; 63:167–173.

79. Saiz-Sanchez D, Ubeda-Bañon I, De la Rosa-Prieto C, Martinez-Marcos A. Differential expression of interneuron populations and correlation with amyloid-β deposition in the olfactory cortex of an AβPP/PS1 transgenic mouse model of Alzheimer's disease. J Alzheimers Dis. 2012; 31:113–129.

81. Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, Carta AR. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson's disease. Eur J Neurosci. 2009; 29:954–963.

82. Seo Y, Kim HS, Kang I, Choi SW, Shin TH, Shin JH, Lee BC, Lee JY, Kim JJ, Kook MG, Kang KS. Cathepsin S contributes to microglia-mediated olfactory dysfunction through the regulation of Cx3cl1-Cx3cr1 axis in a Niemann-Pick disease type C1 model. Glia. 2016; 64:2291–2305.

83. Seo Y, Kim HS, Shin Y, Kang I, Choi SW, Yu KR, Seo KW, Kang KS. Excessive microglial activation aggravates olfactory dysfunction by impeding the survival of newborn neurons in the olfactory bulb of Niemann-Pick disease type C1 mice. Biochim Biophys Acta. 2014; 1842:2193–2203.

84. Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010; 7:483–495.

85. Sohrabi HR, Bates KA, Weinborn MG, Johnston AN, Bahramian A, Taddei K, Laws SM, Rodrigues M, Morici M, Howard M, Martins G, Mackay-Sim A, Gandy SE, Martins RN. Olfactory discrimination predicts cognitive decline among community-dwelling older adults. Transl Psychiatry. 2012; 2:e118.

86. Steinbach S, Staudenmaier R, Hummel T, Arnold W. [Loss of olfaction with aging: a frequent disorder receiving little attention]. Z Gerontol Geriatr. 2008; 41:394–402. German.
87. Sultan B, May LA, Lane AP. The role of TNF-α in inflammatory olfactory loss. Laryngoscope. 2011; 121:2481–2486.

88. Sunderman FW Jr. Nasal toxicity, carcinogenicity, and olfactory uptake of metals. Ann Clin Lab Sci. 2001; 31:3–24.
89. Suzukawa K, Kondo K, Kanaya K, Sakamoto T, Watanabe K, Ushio M, Kaga K, Yamasoba T. Age-related changes of the regeneration mode in the mouse peripheral olfactory system following olfactotoxic drug methimazole-induced damage. J Comp Neurol. 2011; 519:2154–2174.

90. Tang Y, Li H, Liu JP. Niemann-pick disease type C: from molecule to clinic. Clin Exp Pharmacol Physiol. 2010; 37:132–140.

91. Taylor TN, Caudle WM, Shepherd KR, Noorian A, Jackson CR, Iuvone PM, Weinshenker D, Greene JG, Miller GW. Nonmotor symptoms of Parkinson's disease revealed in an animal model with reduced monoamine storage capacity. J Neurosci. 2009; 29:8103–8113.

92. Tepavčević V, Lazarini F, Alfaro-Cervello C, Kerninon C, Yoshikawa K, Garcia-Verdugo JM, Lledo PM, Nait-Oumesmar B, Baron-Van Evercooren A. Inflammation-induced subventricular zone dysfunction leads to olfactory deficits in a targeted mouse model of multiple sclerosis. J Clin Invest. 2011; 121:4722–4734.

93. Tremblay MÈ, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011; 31:16064–16069.

94. Turner JH, Liang KL, May L, Lane AP. Tumor necrosis factor alpha inhibits olfactory regeneration in a transgenic model of chronic rhinosinusitis-associated olfactory loss. Am J Rhinol Allergy. 2010; 24:336–340.

95. Van Dijck A, Vloeberghs E, Van Dam D, Staufenbiel M, De Deyn PP. Evaluation of the APP23-model for Alzheimer's disease in the odour paired-associate test for hippocampusdependent memory. Behav Brain Res. 2008; 190:147–151.

97. Velayudhan L, Lovestone S. Smell identification test as a treatment response marker in patients with Alzheimer disease receiving donepezil. J Clin Psychopharmacol. 2009; 29:387–390.

98. von Bernhardi R, Eugenín J. Microglial reactivity to beta-amyloid is modulated by astrocytes and proinflammatory factors. Brain Res. 2004; 1025:186–193.

99. Wang J, Eslinger PJ, Doty RL, Zimmerman EK, Grunfeld R, Sun X, Meadowcroft MD, Connor JR, Price JL, Smith MB, Yang QX. Olfactory deficit detected by fMRI in early Alzheimer's disease. Brain Res. 2010; 1357:184–194.

100. Wesson DW, Levy E, Nixon RA, Wilson DA. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer's disease mouse model. J Neurosci. 2010; 30:505–514.

101. Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer's disease. Ann N Y Acad Sci. 2009; 1170:730–735.

102. Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relationship between cerebral Alzheimer's disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry. 2007; 78:30–35.

103. Witt RM, Galligan MM, Despinoy JR, Segal R. Olfactory behavioral testing in the adult mouse. J Vis Exp. 2009; (23):e949.

104. Wu N, Rao X, Gao Y, Wang J, Xu F. Amyloid-β deposition and olfactory dysfunction in an Alzheimer's disease model. J Alzheimers Dis. 2013; 37:699–712.

105. Xie F, Fang C, Schnittke N, Schwob JE, Ding X. Mechanisms of permanent loss of olfactory receptor neurons induced by the herbicide 2,6-dichlorobenzonitrile: effects on stem cells and noninvolvement of acute induction of the inflammatory cytokine IL-6. Toxicol Appl Pharmacol. 2013; 272:598–607.

106. Xu L, He D, Bai Y. Microglia-mediated inflammation and neurodegenerative disease. Mol Neurobiol. 2016; 53:6709–6715.

107. Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009; 48:Unit 8.24.

108. Young JW, Sharkey J, Finlayson K. Progressive impairment in olfactory working memory in a mouse model of Mild Cognitive Impairment. Neurobiol Aging. 2009; 30:1430–1443.

109. Zhang S, Xiao Q, Le W. Olfactory dysfunction and neurotransmitter disturbance in olfactory bulb of transgenic mice expressing human A53T mutant alpha-synuclein. PLoS One. 2015; 10:e0119928.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download