1. Campagnolo ER, Rankin JT, Daverio SA, Hunt EA, Lute JR, Tewari D, Acland HM, Ostrowski SR, Moll ME, Urdaneta VV, Ostroff SM. Fatal pandemic (H1N1) 2009 influenza A virus infection in a Pennsylvania domestic cat. Zoonoses Public Health. 2011. 58:500–507.

2. Forrest HL, Kim JK, Webster RG. Virus shedding and potential for interspecies waterborne transmission of highly pathogenic H5N1 influenza virus in sparrows and chickens. J Virol. 2010. 84:3718–3720.

3. Guan Y, Peiris JSM, Lipatov AS, Ellis TM, Dyrting KC, Krauss S, Zhang LJ, Webster RG, Shortridge KF. Emergence of multiple genotypes of H5N1 avian influenza viruses in Hong Kong SAR. Proc Natl Acad Sci USA. 2002. 99:8950–8955.

4. Humberd J, Guan Y, Webster RG. Comparison of the replication of influenza A viruses in Chinese ring-necked pheasants and chukar partridges. J Virol. 2006. 80:2151–2161.

5. Ilyushina NA, Kim JK, Negovetich NJ, Choi YK, Lang V, Bovin NV, Forrest HL, Song MS, Pascua PNQ, Kim CJ, Webster RG, Webby RJ. Extensive mammalian ancestry of pandemic (H1N1) 2009 virus. Emerg Infect Dis. 2010. 16:314–317.

6. Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, Muramoto Y, Tamura D, Sakai-Tagawa Y, Noda T, Sakabe S, Imai M, Hatta Y, Watanabe S, Li C, Yamada S, Fujii K, Murakami S, Imai H, Kakugawa S, Ito M, Takano R, Iwatsuki-Horimoto K, Shimojima M, Horimoto T, Goto H, Takahashi K, Makino A, Ishigaki H, Nakayama M, Okamatsu M, Takahashi K, Warshauer D, Shult PA, Saito R, Suzuki H, Furuta Y, Yamashita M, Mitamura K, Nakano K, Nakamura M, Brockman-Schneider R, Mitamura H, Yamazaki M, Sugaya N, Suresh M, Ozawa M, Neumann G, Gern J, Kida H, Ogasawara K, Kawaoka Y.
In vitro and
in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009. 460:1021–1025.

7. Jones YL, Swayne DE. Comparative pathobiology of low and high pathogenicity H7N3 Chilean avian influenza viruses in chickens. Avian Dis. 2004. 48:119–128.

8. Kitikoon P, Nilubol D, Erickson BJ, Janke BH, Hoover TC, Sornsen SA, Thacker EL. The immune response and maternal antibody interference to a heterologous H1N1 swine influenza virus infection following vaccination. Vet Immunol Immunopathol. 2006. 112:117–128.

9. Kitikoon P, Sreta D, Na Ayudhya SN, Wongphatcharachai M, Lapkuntod J, Prakairungnamthip D, Bunpapong N, Suradhat S, Thanawongnuwech R, Amonsin A. Brief report: molecular characterization of a novel reassorted pandemic H1N1 2009 in Thai pigs. Virus Genes. 2011. 43:1–5.

10. Liu M, He S, Walker D, Zhou N, Perez DR, Mo B, Li F, Huang X, Webster RG, Webby RJ. The influenza virus gene pool in a poultry market in South central China. Virology. 2003. 305:267–275.

11. Makarova NV, Ozaki H, Kida H, Webster RG, Perez DR. Replication and transmission of influenza viruses in Japanese quail. Virology. 2003. 310:8–15.

12. Mathieu C, Moreno V, Retamal P, Gonzalez A, Rivera A, Fuller J, Jara C, Lecocq C, Rojas M, Garcia A, Vasquez M, Agredo M, Gutiérrez C, Escobar H, Fasce R, Mora J, Garcia J, Fernández J, Ternicier C, Avalos P. Pandemic (H1N1) 2009 in breeding turkeys, Valparaiso, Chile. Emerg Infect Dis. 2010. 16:709–711.

13. Mo IP, Brugh M, Fletcher OJ, Rowland GN, Swayne DE. Comparative pathology of chickens experimentally inoculated with avian influenza viruses of low and high pathogenicity. Avian Dis. 1997. 41:125–136.

14. Mutinelli F, Capua I, Terregino C, Cattoli G. Clinical, gross, and microscopic findings in different avian species naturally infected during the H7N1 low- and high-pathogenicity avian influenza epidemics in Italy during 1999 and 2000. Avian Dis. 2003. 47:844–848.

15. Nardelli L, Rinaldi A, Pereira HG, Mandelli G. Influenza virus infections in Japanese quails. Arch Exp Veterinarmed. 1970. 24:231–249.
16. Nfon C, Berhane Y, Zhang S, Handel K, Labrecque O, Pasick J. Molecular and antigenic characterization of triple-reassortant H3N2 swine influenza viruses isolated from pigs, turkey and quail in Canada. Transbound Emerg Dis. 2011. 58:394–401.

17. Nili H, Asasi K, Dadras H, Ebrahimi M. Pathobiology of H9N2 avian influenza virus in Japanese quail (
Coturnix coturnix japonica). Avian Dis. 2007. 51:1 Suppl. 390–392.

18. Pereda A, Cappuccio J, Quiroga MA, Baumeister E, Insarralde L, Ibar M, Sanguinetti R, Cannilla ML, Franzese D, Escobar Cabrera OE, Craig MI, Rimondi A, Machuca M, Debenedetti RT, Zenobi C, Barral L, Balzano R, Capalbo S, Risso A, Perfumo CJ. Pandemic (H1N1) 2009 outbreak on pig farm, Argentina. Emerg Infect Dis. 2010. 16:304–307.

19. Perez DR, Webby RJ, Hoffmann E, Webster RG. Land-based birds as potential disseminators of avian/mammalian reassortant influenza A viruses. Avian Dis. 2003. 47:3 Suppl. 1114–1117.

20. Pillai SPS, Pantin-Jackwood M, Suarez DL, Saif YM, Lee CW. Pathobiological characterization of low-pathogenicity H5 avian influenza viruses of diverse origins in chickens, ducks and turkeys. Arch Virol. 2010. 155:1439–1451.

21. Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938. 27:493–497.
22. Saito T, Watanabe C, Takemae N, Chaisingh A, Uchida Y, Buranathai C, Suzuki H, Okamatsu M, Imada T, Parchariyanon S, Traiwanatam N, Yamaguchi S. Pathogenicity of highly pathogenic avian influenza viruses of H5N1 subtype isolated in Thailand for different poultry species. Vet Microbiol. 2009. 133:65–74.

23. Smith GJD, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JSM, Guan Y, Rambaut A. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009. 459:1122–1125.

24. Sreta D, Kedkovid R, Tuamsang S, Kitikoon P, Thanawongnuwech R. Pathogenesis of swine influenza virus (Thai isolates) in weanling pigs: an experimental trial. Virol J. 2009. 6:34.

25. Sreta D, Tantawet S, Na Ayudhya SN, Thontiravong A, Wongphatcharachai M, Lapkuntod J, Bunpapong N, Tuanudom R, Suradhat S, Vimolket L, Poovorawan Y, Thanawongnuwech R, Amonsin A, Kitikoon P. Pandemic (H1N1) 2009 virus on commercial swine farm, Thailand. Emerg Infect Dis. 2010. 16:1587–1590.

26. Suzuki Y, Ito T, Suzuki T, Holland RE Jr, Chambers TM, Kiso M, Ishida H, Kawaoka Y. Sialic acid species as a determinant of the host range of influenza A viruses. J Virol. 2000. 74:11825–11831.

27. Swayne DE, Pantin-Jackwood M, Kapczynski D, Spackman E, Suarez DL. Susceptibility of poultry to pandemic (H1N1) 2009 Virus. Emerg Infect Dis. 2009. 15:2061–2063.

28. Tashiro M, Reinacher M, Rott R. Aggravation of pathogenicity of an avian influenza virus by adaptation to quails. Arch Virol. 1987. 93:81–95.

29. Van Reeth K. Avian and swine influenza viruses: our current understanding of the zoonotic risk. Vet Res. 2007. 38:243–260.

30. Wan H, Perez DR. Quail carry sialic acid receptors compatible with binding of avian and human influenza viruses. Virology. 2006. 346:278–286.

31. Webster RG, Guan Y, Peiris M, Walker D, Krauss S, Zhou NN, Govorkova EA, Ellis TM, Dyrting KC, Sit T, Perez DR, Shortridge KF. Characterization of H5N1 influenza viruses that continue to circulate in geese in southeastern China. J Virol. 2002. 76:118–126.

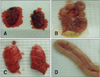


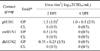
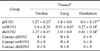




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download