Abstract
This study was designed to evaluate the reproductive performance of Japanese black cows following the 3rd injection of gonadotropin releasing hormone (GnRH) analogue administered concurrently with Ovsynch-based treatment on day 6 (day 1 = the day of ovulation). In Experiment 1, 12 cows were allocated into three groups: a control group that was subjected to Ovsynch treatment and then injected with a placebo on day 6; group 1 (Ovsynch + GnRH), which was subjected to Ovsynch treatment and was injected with GnRH analogue on day 6, and group 2 (Ovsynch + controlled internal drug-release (CIDR) + GnRH), which received Ovsynch-CIDR treatment and was injected with GnRH analogue on day 6. Blood collection and ultrasonographic observation of the ovaries were conducted daily. Both treatments induced the formation of an accessory corpus luteum and significantly increased the cross-sectional area of the luteal tissue when compared to the control. However, plasma progesterone (P4) was significantly higher in the treatment groups than in the control group on days 11, 12, 17 and 18 in the group 1 and from day 10 to 21 in the group 2. In Experiment 2, 41 cows were assigned to the same three groups described above and then artificially inseminated on day 1. The pregnancy rates on day 45 did not differ among groups. In conclusion, administration of GnRH analogue on day 6 following Ovsynch-based treatment did not improve the reproductive performance of Japanese black cows, even though the P4 concentration was higher in groups that received the GnRH.
Early embryo death is considered to be one of the most important factors affecting fertility in cattle. For example, a previously conducted study found that, although the fertilization rate immediately following insemination of non-lactating beef cows was 100%, the survival rate of the embryos during days 14 to 16 was only 82.4% [17]. One of the factors that lead to early embryonic mortality in cows is a lower plasma progesterone (P4) concentration during the post insemination period [15]. Progesterone is involved in stimulation of a variety of endometrial secretions that are necessary for successful development of the embryo in the uterine lumen [10]. It has been reported that a suboptimum P4 concentration is correlated with the failure of embryo implantation and low fertility in cows [16]. However, it has been reported that bovine elongated embryos (well developed) are able to produce sufficient quantities of interferon-τ that are capable to prevent luteolytic PGF2α secretion. Whereas poor developed embryos are associated with low interferon-τ production, failed inhibition of luteolysis and embryo loss [15]. Specifically, the production of interferon-τ by bovine embryos tended to be greater on day 18, when the P4 concentration had increased in response to the induction of an accessory corpus luteum (CL) [12]. In addition, the concentration of embryonic interferon-τ has been found to increase significantly on day 16 when progesterone was administered from days 5 to 9 [15]. Taken together, these findings indicate that increasing the P4 concentration by administering exogenous progesterone supplements using a controlled internal drug-release (CIDR) [7,25] or progesterone releasing intravaginal device (PRID) [13,29], or by feeding the animal progesterone [23,25] may improve fertility and assist in the maintenance of pregnancy in cows. Furthermore, it is known that induction of ovulation and the consequent formation of an accessory CL following treatment with human chorionic gonadotropin (hCG) or gonadotropin releasing hormone (GnRH) at either day 5, 6, 11 or 14 of the estrous cycle will result in an increase in P4 concentration, thereby improving fertility [27]. Indeed, it has been reported that cows that have an additional accessory CL are 8.3 times less likely to experience fetal loss than cows that have only a single CL [14].
This study was conducted to evaluate the effects of injecting GnRH analogue on day 6 of the estrous cycle on the reproductive performance of Japanese black cows that were currently undergoing Ovsynch-based treatment. Specifically, the P4 profile and its relationship with the cross sectional area of the luteal tissue in non-inseminated cows, as well as the conception rate in artificially inseminated cows was evaluated.
Fig. 1 shows the protocol for this experiment. The goal of Experiment 1 was to examine the P4 profile and its relationship with the cross sectional area of luteal tissue in non-inseminated cows subjected to two different estrus synchronization programs. The animals used for Experiment 1 included 12 multiparous Japanese black cows that had lapsed approximately 40 days since their last parturition and had body condition scores (BCS; point scale from 1 to 5) [6] between 3.0 and 4.0. All animals were kept outdoors, fed hay and concentrate twice daily, and provided with water ad libitum.
The estrous cycles of the cows were synchronized by a single intramuscular injection of 500 µg of prostaglandin F2α (PGF) analogue (Resipron-C; Teikoku Zoki, Japan), after which ovulation was confirmed by real-time ultrasonography (Aloka, Japan). When no ovulation was confirmed, the cows received a 2nd injection of PGF analogue 11 days after the 1st PGF analogue injection. Next, the 12 cows were randomly allocated to three experimental groups. In group 1 (Ovsynch + GnRH), the cows were synchronized with Ovsynch [20] and then injected with 100 µg of GnRH analogue (Buserelin; Teikoku Zoki, Japan) on day 6 (the 2nd GnRH analogue injection of Ovsynch was considered as day 0); In group 2 (Ovsynch + CIDR + GnRH), the cows were synchronized with Ovsynch + CIDR (Pfizer, Japan) and then injected with 100 µg of GnRH analogue on day 6. The remaining cows (the control) were synchronized with Ovsynch and then injected with physiological saline (placebo).
Blood samples were collected once a day before starting the ultrasonographical observation into heparinized tubes from the jugular vein of each animal and centrifuged at 1,670 g for 20 min at 4℃. The plasma samples were then stored at -20℃ until hormone analysis was performed. Double antibody radioimmunoassay was used to determine the concentrations of plasma P4 using antisera to progesterone (GDN#337) [26], and the intra- and interassay coefficients for progesterone were found to be 4.2 and 8.0%, respectively.
To monitor the growth and regression of follicles and CL, the ovaries of all cows were scanned once a day throughout the experimental period (from the initial day of synchronization until next spontaneous ovulation) using real-time ultrasonography. The cross-sectional areas (mm2) of the dominant follicle (DF) and the luteal tissue were then determined using the following formula: Elliptical area = π (π equal 3.14) × (diameter a/2) × (diameter b/2), and the total CL cross-sectional area per cow was calculated by summation of the CL cross-sectional area (CL c-s area) in both ovaries.
Fig. 2 shows the protocol of Experiment 2. This study was conducted to examine the effect of day 6 injection of GnRH analogue in two estrus synchronization methods on the pregnancy rate of Japanese black cows. To accomplish this, a total of 41 multiparous Japanese black cows were randomly assigned into the same three experimental groups used in experiment 1 and then inseminated 16 to 20 h after the 2nd GnRH analogue injection. Rectal palpation was then conducted on day 45 to determine if the cows were pregnant. Experiment 2 was conducted at three private beef cattle farms located near our laboratory in Kagoshima prefecture. All cows were housed in tie-stalls.
ANOVA (Dunnett's method) was used to determine if the P4 concentrations and CL c-s areas differed between the treatment groups and the control group. In addition, the differences in the CL c-s areas as a result of the different size of the dominant follicles were evaluated using ANOVA (Sheffe's test for multiple comparison). Finally, a student's t test was used to compare the diameters of the dominant follicles among groups on day 0 and one day before the next spontaneous ovulation, and the pregnancy rate among different groups on day 45 was compared using the χ square test. For all tests, a p-value of less than 0.05 was considered to be statistically significant.
All cows ovulated within 3 to 5 days of administration of the 1st or 2nd injection of the PGF analogue. Ovulation of the DF after administration of the 1st GnRH analogue to the cows synchronized using PGF occurred in 75% of cows in the control group, 50% of the cows in the Ovsynch + GnRH group and 100% of cows in the Ovsynch + CIDR + GnRH group. In contrast, ovulation following a 2nd GnRH analogue injection that was administered 9 days after the 1st GnRH injection occurred in 100% of cows in all three groups. In addition, the Ovsynch + GnRH group and the Ovsynch + CIDR + GnRH group ovulated and formed an accessory CL after administration of the 3rd GnRH analogue on day 6. Furthermore, a small CL was formed after induced ovulation of a small DF (less than 10 mm in diameter), regardless of the synchronization method used. Conversely, a large CL was formed after induced ovulation of a large DF (greater than 13 mm in diameter). As shown in Fig. 3, there is a positive correlation between the cross-sectional area of the DF one day before ovulation and the cross-sectional area of luteal tissue on day 12. The DF of each group has been classified into one of the following groups based on its size: large (>13 mm), medium (<13 mm >11 mm), and small (<11 mm). The correlation between DF size one day prior to ovulation and the CL cross-sectional area on day 12 (mature CL) was 0.96.
Fig. 4 shows the P4 concentrations for all groups. The mean P4 concentration was higher in cows in the Ovsynch + GnRH group and the Ovsynch + CIDR + GnRH group, which had the accessory CL, than in cows in the control group. In addition, the P4 concentrations in the Ovsynch + CIDR + GnRH group were significantly higher than the concentrations in the control group from day 10 to day 21 (p < 0.05). Finally, the P4 concentrations in the Ovsynch + GnRH group were significantly higher than the concentrations in the control group on days 11, 12, 17, and 18 (p < 0.05).
The P4 concentration was found to positively correlated with the total cross-sectional area of the CL in all groups, with correlation coefficients of 0.89, 0.95 and 0.87 being observed for the Ovsynch + GnRH group, the Ovsynch + CIDR + GnRH group and the control group, respectively (Figs. 5A, B and C). In addition, there was a significant difference in the cross-sectional area of luteal tissue in the control and treatment groups from days 12 to 22. Finally, as shown in Fig. 6, there was no difference in the cross-sectional area of the luteal tissue between the Ovsynch + GnRH group and the Ovsynch + CIDR + GnRH group.
The length of the estrous cycle in one of the ovulating cows in the Ovsynch + CIDR + GnRH group was extended to 24 days, while the cycle of another cow in the same group was extended to 25 days. Cows that ovulated spontaneously in the cycle following the Ovsynch treatment had a larger-diameter DF than was observed during the Ovsynch synchronization period for all groups (Table 1); however, this difference was not statistically significant.
This study was designed to examine the effects of treatment with GnRH analogue 6 days after ovulatory synchronization by Ovsynch-based treatments on the pregnancy rate in postpartum multiparous Japanese black cows.
Many studies have reported a relationship between ovarian dynamics, hormonal concentrations and fertility following application of ovulatory synchronization methods. In a study that evaluated ovulatory synchronization methods that did not use an exogenous progesterone source, the P4 concentration prior to estrus synchronization was found to be lower in some cows [22]. In addition, a positive correlation between P4 concentration during the pre-insemination luteal phase and the conception rate has previously been reported [21]. Furthermore, it has been reported that a suboptimum P4 concentration combined with an increasing luteinizing hormone (LH) pulse frequency can induce ovulation of premature oocytes (relatively small ovulatory follicle) [18]. This can affect the embryo quality [1,18], thereby inducing the premature release of PGF2α in the subsequent cycle [24] and negatively affecting fertility.
In this study, a small ovulatory follicle (physiologically immature) resulted in formation of a small CL following induced ovulation. Such a small CL, in turn, resulted in the production of a lower concentration of progesterone than occurs when a normal CL is present [18]. This lower concentration is insufficient to maintain pregnancy in many cases [15]. In addition, it is believed that the low P4 concentration that is observed after insemination is associated with uterine secretion of PGF2α, which may interfere with maternal recognition of pregnancy and result in embryo loss [24].
In the present study, ovulation occurred in 100% of the cows that received both the 2nd and 3rd GnRH injections. This result may have been due to the injection of Buserelin (a potent GnRH analogue designed to induce the release of LH and FSH) and injection at the optimum time [4]. These results agree with the results of previously conducted studies that found a single dose of GnRH agonist administered on day 6 is capable of inducing ovulation, thereby leading to the formation of an accessory CL [11].
The cross-sectional area of the ovulatory follicle one day before ovulation was found to be positively correlated with the maximum cross-sectional area of the CL that was subsequently formed and the P4 concentration, regardless of the ovulation synchronization methods used. This is consistent with the results of a previously conducted study that reported formation of small CL after ovulation of small dominant follicle [28].
It has been reported that pulsatile LH secretion is responsible for early CL development in cows between days 2 and 12, and that this is required for normal progesterone production to occur [19]. In addition, Fraser et al. [8] reported that the most intense angiogenesis of the newly formed CL occurred during the early luteal phase in all mammals, and that this was primarily regulated by LH. Furthermore, Dhali et al. [5] showed that the early stages of CL development continued until days 5 to 6 of the estrous cycle, while a fully functional CL existed approximately at the mid-estrous cycle in Mithun cows (Bos frontalis). These findings suggest that a greater basal LH concentration and frequent low amplitude LH pulses facilitate early CL development. Finally, the results of studies conducted to evaluate ewes indicated that the luteal weight is reduced in the absence of LH support, which leads to a low P4 concentration [9].
In the present study, the post ovulation P4 concentration in the Ovsynch + CIDR + GnRH group tended to be higher than that of the Ovsynch + GnRH group. This may suggest that the rise in the P4 concentration occurred due to the presence of a sufficient concentration of LH following ovulation, and that this was caused by a high pre-ovulatory P4 concentration induced by the Ovsynch + CIDR + GnRH treatment.
In the present study, it was clearly demonstrated that the increased P4 concentration in the Ovsynch + GnRH group and the Ovsynch + CIDR + GnRH group was primarily due to the increased cross-sectional area of the luteal tissue, which resulted from the formation of an accessory CL following the day 6 GnRH analogue injection.
Many studies have been conducted to elucidate the relationship between day 5 or 6 post-insemination injection of GnRH analogue and the increase in conception rate. Some of these studies have found a negative correlation in cows [2,11] and buffalo [3], while others have reported a positive correlation in heat stressed cows [30]. The results of the present study indicate that treatment with GnRH analogue on day 6 in cows synchronized by Ovsynch or Ovsych + CIDR and then subjected to Timed AI (TAI) had no effect on the pregnancy rate. However, these results also suggest that this treatment can maintain the rate of pregnancies at levels higher than 55%.
In conclusion, treatment with GnRH analogue on day 6 after ovulation synchronization using Ovsynch or Ovsych + CIDR increases in the plasma progesterone concentration when compared to controls. This effect was likely due to an increased the total area of luteal tissue that was generated as a result of the formation of an accessory CL. However, these changes did not improve the pregnancy rate in Japanese black cows.
Figures and Tables
Fig. 1
Experiment 1 protocol. All cows were pre-synchronized using single or double intra muscular injection of prostaglandin F2α analogue 11 days apart. Next, 12 cows were randomly allocated into three experimental groups. Ovsynch synchronization was then induced 8 ± 1 days after the last PGf2α injection. The cows in group 1 (Ovsynch + GnRH) were synchronized with Ovsynch followed by injection of 100 µg of GnRH analogue on day 6 (the day on which the 2nd GnRH analogue injection of Ovsynch protocol was administered was considered to be day 0). The cows in group 2 (Ovsynch + CIDR + GnRH) were synchronized with Ovsynch + CIDR followed by injection of 100 µg of GnRH analogue on by day 6. The remaining cows (Control) were synchronized with Ovsynch followed by injection with physiological saline (placebo) on day 6. All cows were monitored daily by real time ultrasonography until the next estrus. In addition, blood samples were collected daily from the time at which Ovsynch treatment began until ovulation in the first estrous cycle following the administration of Ovsynch. PGF; prostaglandin F2α. GnRH; gonadotropin releasing hormone. CIDR; controlled internal drug-release.
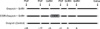
Fig. 2
Experiment 2 protocol. Multiparous Japanese black cows (n = 41) were randomly assigned into the three experimental groups (Ovsynch, Ovsynch + GnRH, and Ovsynch + CIDR + GnRH) that are described in the experiment 1 protocol, and then inseminated 16 to 20 h after receiving the 2nd GnRH analogue injection (day 0). On day 45, the cows were evaluated by palpation of the rectum to determine if they were pregnant.

Fig. 3
Cross-sectional area of dominant Follicles (DF) of different size and the cross-section area of the subsequently formed corpus luteum (CL). Values shown are the mean ± SD. Letters (a, b) and (a, c) on the same day indicate a statistical difference (p < 0.05). The DF at one day before ovulation was assigned to one of the following 3 categories based on its diameter, regardless of the group it was from: large (>13 mm), medium (<13 mm >11 mm), and small (<11 mm).

Fig. 4
Progesterone (P4) concentration of the control (Ovsynch) and experimental groups (Ovsynch + GnRH, and Ovsynch + CIDR + GnRH). Values shown are mean ± SD. Letter (a,b) indicates the value is significantly different from (c) (p < 0.05) on the same day.

Fig. 5
(A) Corpus luteum (CL) cross-sectional area (c-s area) and progesterone concentration of the control group (Ovsynch). Values are the mean ± SD. The progesterone (P4) concentration was positively correlated with the cross-sectional area of CL (r = 0.87). (B) Summation of the CL cross-sectional area (2 CL) and progesterone concentration of the Ovsynch + GnRH group. Values are the mean ± SD. The progesterone (P4) concentration was positively correlated with the cross-sectional area of the CL (r = 0.89). (C) Summation of the CL cross-sectional area (2 CL) and the progesterone concentration of the Ovsynch + CIDR + GnRH group. Values are the mean ± SD. The progesterone (P4) concentration was positively correlated with the cross-sectional area of the CL (r = 0.95).

Fig. 6
Cross-sectional area of the corpus luteum (CL) from the control (Ovsynch) and experimental groups (Ovsynch + GnRH, and Ovsynch + CIDR + GnRH). Value are the mean ± SD. (a, b) differ significantly from (c) from day 11 to day 23 (p < 0.05).

Acknowledgments
We are grateful to the staff of the IRIKI farm of Kagoshima University and the students at the theriogenology laboratory of Kagoshima University for their help and assistance. In addition, we thank Professor Taya at the Tokyo University of Agriculture and Technology for his donation of progesterone antisera, and Professor Okamoto of Kagoshima University for his assistance in the data analysis.
References
1. Ahmad N, Schrick FN, Butcher RL, Inskeep EK. Effect of persistent follicles on early embryonic losses in beef cows. Biol Reprod. 1995. 52:1129–1135.
2. Bartolome JA, Melendez P, Kelbert D, Swift K, McHale J, Hernandez J, Silvestre F, Risco CA, Arteche ACM, Thatcher WW, Archbald LF. Strategic use of Gonadotrophin-releasing hormone (GnRH) to increase pregnancy rate and reduce pregnancy loss in lactating dairy cows subjected to synchronization of ovulation and timed insemination. Theriogenology. 2005. 63:1026–1037.

3. Campanile G, Di Palo R, Neglia G, Vecchio D, Gasparrini B, Prandi A, Galiero G, D'Occhio MJ. Corpus luteum function and embryonic mortality in buffaloes treated with a GnRH agonist, hCG and progesterone. Theriogenology. 2007. 67:1393–1398.

4. Chenault JR, Kratzer DD, Rzepkowski RA, Goodwin MC. LH and FSH response of Holstein heifers to fertirelin acetate, gonadorelin and buserelin. Theriogenology. 1990. 34:81–98.

5. Dhali A, Mishra DP, Mech A, Karunakaran M, Rajkhowa C. Role of LH and prostaglandin F2α on the development and regression of corpus luteum in mithun (Bos frontalis) estrous cycle. Gen Comp Endocrinol. 2006. 149:173–181.

6. Edmonson AJ, Lean IJ, Weaver LD, Farver T, Webster G. A body condition scoring chart for Holstein dairy cows. J Dairy Sci. 1989. 72:68–78.

7. El-Zarkouny SZ, Cartmill JA, Hensley BA, Stevenson JS. Pregnancy in dairy cows after synchronized ovulation regimens with or without presynchronization and progesterone. J Dairy Sci. 2004. 87:1024–1037.

8. Fraser HM, Wulff C. Angiogenesis in the corpus luteum. Reprod Biol Endocrinol. 2003. 1:88–96.
9. Fuller GB, Hansel W. Regression of sheep corpora lutea after treatment with antibovine luteinizing hormone. J Anim Sci. 1970. 31:99–103.

10. Geisert RD, Morgan GL, Short EC Jr, Zavy MT. Endocrine events associated with endometrial function and conceptus development in cattle. Reprod Fertil Dev. 1992. 4:301–305.

11. Howard JM, Manzo R, Dalton JC, Frago F, Ahmadzadeh A. Conception rates and serum progesterone concentration in dairy cattle administered gonadotropin releasing hormone 5 days after artificial insemination. Anim Reprod Sci. 2006. 95:224–233.

12. Kerbler TL, Buhr MM, Jordan LT, Leslie KE, Walton JS. Relationship between maternal plasma progesterone concentration and interferon-tau synthesis by the conceptus in cattle. Theriogenology. 1997. 47:703–714.

13. Kuroiwa T, Ishibashi A, Fukuda M, Kim S, Tanaka T, Kamomae H. Estrus synchronization and conception rate after a progesterone releasing intravaginal device (PRID) treatment from the early luteal phase in heifers. J Reprod Dev. 2005. 51:669–673.

14. López-Gatius F, Santolaria P, Yániz J, Rutllant J, López-Béjar M. Factors affecting pregnancy loss from gestation day 38 to 90 in lactating dairy cows from a single herd. Theriogenology. 2002. 57:1251–1261.

15. Mann GE, Lamming GE. The influence of progesterone during early pregnancy in cattle. Reprod Domest Anim. 1999. 34:269–274.

16. Mann GE, Lamming GE, Robinson RS, Wathes DC. The regulation of interferon-tau production and uterine hormone receptors during early pregnancy. J Reprod Fertil Suppl. 1999. 54:317–328.
17. Maurer RR, Chenault JR. Fertilization failure and embryonic mortality in parous and non-parous beef cattle. J Anim Sci. 1983. 56:1186–1189.

18. Mussard ML, Burke CR, Behlke EJ, Gasser CL, Day ML. Influence of premature induction of a luteinizing hormone surge with gonadotropin-releasing hormone on ovulation, luteal function, and fertility in cattle. J Anim Sci. 2007. 85:937–943.

19. Peters KE, Bergfeld EG, Cupp AS, Kojima FN, Mariscal V, Sanchez T, Wehrman ME, Grotjan HE, Hamernik DL, Kittok RJ, Kinder JE. Luteinizing hormone has a role in development of fully functional corpora lutea (CL) but is not required to maintain CL function in heifers. Biol Reprod. 1994. 51:1248–1254.

20. Pursley JR, Mee MO, Wiltbank MC. Synchronization of ovulation in dairy cows using PGF2α and GnRH. Theriogenology. 1995. 44:915–923.

21. Rosenberg M, Kaim M, Herz Z, Folman Y. Comparison of methods for the synchronization of estrous cycles in dairy cows. 1. Effects on plasma progesterone and manifestation of estrus. J Dairy Sci. 1990. 73:2807–2816.

22. Santos JEP, Thatcher WW, Chebel RC, Cerri RLA, Galvão KN. The effect of embryonic death rates in cattle on the efficacy of estrus synchronization programs. Anim Reprod Sci. 2004. 82-83:513–535.

23. Schafer DJ, Bader JF, Meyer JP, Haden JK, Ellersieck MR, Lucy MC, Smith MF, Patterson DJ. Comparison of progestin-based protocols to synchronize estrus and ovulation before fixed-time artificial insemination in postpartum beef cows. J Anim Sci. 2007. 85:1940–1945.

24. Shaham-Albalancy A, Folman Y, Kaim M, Rosenberg M, Wolfenson D. Delayed effect of low progesterone concentrations on bovine uterine PGF2α secretion in the subsequent oestrous cycle. Reproduction. 2001. 122:643–648.
25. Tauck SA, Wilkinson JR, Olsen JR, Janitell JN, Berardinelli JG. Comparison of controlled internal drug release device and melengesterol acetate as progestin sources in an estrous synchronization protocol for beef heifers. Theriogenology. 2007. 68:162–167.

26. Taya K, Watanabe G, Sasamoto S. Radioimmunoassay for progesterone, testosterone and estradiol-17 beta using 125I-iodohistamine radioligands. Jpn J Anim Reprod. 1985. 31:186–197.

27. Thatcher WW, Bilby TR, Bartolome JA, Silvestre F, Staples CR, Santos JEP. Strategies for improving fertility in the modern dairy cow. Theriogenology. 2006. 65:30–44.

28. Vasconcelos JLM, Sartori R, Oliveira HN, Guenther JG, Wiltbank MC. Reduction in size of the ovulatory follicle reduces subsequent luteal size and pregnancy rate. Theriogenology. 2001. 56:307–314.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download