Abstract
This study aimed to elucidate the effect of tryptophan (Trp) on gut hormone secretion as well as the roles of the calcium-sensing receptor (CaSR) and its downstream signaling pathway in gut hormone secretion by assessing swine duodenal perfusion in vitro. Swine duodenum was perfused with Krebs-Henseleit buffer as a basal solution. Various concentrations (0, 10, and 20 mM) of Trp were applied to investigate its effect on gut hormone secretion. A CaSR antagonist was used to detect the involvement of CaSR and its signal molecules. The 20 mM Trp concentration promoted the secretion of cholecystokinin (CCK) and glucose-dependent insulinotropic peptide (GIP), elevated the mRNA level of CaSR, and upregulated the protein levels of CaSR, protein kinase C (PKC), and inositol trisphosphate receptor (IP3R). However, NPS 2143, an inhibitor of CaSR, attenuated the CCK and GIP release, reduced the mRNA level of CaSR, and decreased the protein levels of CaSR, PKC, and IP3R with 20 mM Trp perfusion. The results indicate that CCK and GIP secretion can be induced by Trp in swine duodenum in vitro, and the effect is mediated by CaSR and its downstream signal molecules PKC and IP3R.
Enteroendocrine cells (EECs) are widely distributed in the epithelial layer of the gastrointestinal (GI) tract in animals and humans, and a range of gut hormones are produced and secreted by EECs. For example, the gut hormone cholecystokinin (CCK) is produced by I cells, whereas glucose-dependent insulinotropic peptide (GIP) is secreted from intestinal enteroendocrine K cells. These hormones not only target the brain to regulate appetite but also have a role in energy homeostasis and in homeostasis of body adiposity [1819]. Thus, these hormones can also be used as a discreet endogenous system to treat obesity with minimal side effects [20]. Recent studies have shown that nutrients in food/feed, including proteins and amino acids, fatty acids, and glucose, can be detected by EECs to induce the secretion of gut hormones [182223]. It has also been reported that proteins and amino acids induce the secretion of CCK and GIP in the gut of humans, rats, and pigs [6121317].
The calcium-sensing receptor (CaSR), a member of a family of coupled C G-protein receptors, is present in many tissues and organs, including myocardial tissue, the nervous system, and kidney and bone in rodents and humans [4], and it is also widely expressed along the lumen of the GI tract, including the esophagus, stomach, small intestine, and large intestine (colon). CaSR has an important role in sensing nutrients, releasing gut hormones, and regulating the digestion and absorption of nutrients [48]. Moreover, it has been reported that CaSR can sense extracellular aromatic amino acids and mediate CCK and glucagon-like peptide 1 (GLP-1) secretion [15]. Aromatic amino acids, including L-phenylalanine (Phe) and tryptophan (Trp), are the most effective stimulants of secretion of CCK and GIP in rodents and humans [91524]. Perfusion with 50 mM Trp has been demonstrated to induce the secretion of CCK and GLP-1 in the small intestine of rats, and that effect was enhanced by the CaSR agonist NPS R568 but was inhibited by the CaSR antagonists NPS 2143 and Calhex 231 [15]. Another in-vitro study on mice reported that 10 mM and 20 mM of Trp induced a dose-dependent CCK secretion from a mucosal cell culture of the proximal small intestine, and the effect was blocked by Calhex 231 [26]. These investigations suggest that CaSR mediates the secretion of gut hormones induced by Trp in rodents. Furthermore, these studies proposed that the secretion of gut hormones mediated by the activation of CaSR was regulated by the stimulation of the downstream signal molecules protein kinase C (PKC) and inositol trisphosphate receptor (IP3R) of CaSR and by the mobilization of intracellular Ca2+ ([Ca2+]i) in the EECs of rat and mouse STC-1 cells [1527]. To date, only one study on pigs has reported that the intraduodenal infusion of casein hydrolysate provokes a transient increase of CCK in plasma during 30 min of perfusion [6]. However, nothing has been reported about the mediating effect of the activation of CaSR and its downstream signal mechanisms on Trp-induced gut hormone secretion in pigs.
We postulated that Trp could induce CCK and GIP secretion in swine, and that such secretion is mediated and regulated by the activation of CaSR and its downstream signaling pathway. Therefore, a perfusion system was applied in vitro to determine the effect of Trp on the secretion of CCK and GIP in pig duodenum and to explore the involvement of CaSR and its downstream signaling pathway in the release of CCK and GIP by using a CaSR inhibitor.
The entire intestine was removed from healthy pigs after they were killed and their bellies opened at a local abattoir. Approximately 4 to 5 cm of the proximal duodenum was collected in a conical centrifuge tube containing fresh and sterile Krebs-Henseleit buffer (KHB) solution at 37℃ and transported to the laboratory within 30 min for perfusion. The KHB solution was formulated as described previously [2]; it consisted of 136.87 mM NaCl, 4.02 mM KCl, 2.10 mM MgCl2, 1.80 mM CaCl2, and 25.18 mM HEPES (pH 7.2). The duodenum tissue was removed from the conical centrifuge tube and rinsed several times with 0.01M phosphate buffered saline (PBS) at 37℃. The PBS solution consisted of 2.68 mM KCl, 136.87 mM NaCl, 1.76 mM KH2PO4, and 10 mM Na2HPO4·12H2O (pH 7.2). The tissue samples were then cut into approximately 1 to 2 mm pieces using a sterile surgical blade on a glass plate. After fully mixing the pieces that were cut, the duodenum pieces were assigned to a chamber (400 mg/chamber) of the perfusion system in a water bath at 37℃. All sampling and experimental procedures were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University (IACUC No. SYXK (Su) 2015-0656).
The perfusion system described by Badger [1] and later modified by Hansen and Conn [7] and Tan et al. [25] was used in the current study. Panel A in Fig. 1 depicts the perfusion system, which is composed of an oxygen bomb, a constant-flow pump, a media reservoir, a water bath, a tissue chamber, and a silica-gel pipe. A sterile single-use 5-mL syringe was used for construction of the chamber (panel B in Fig. 1). Next to the rubber plunger, the syringe was fitted with a nylon filter platform (400 mesh, 31 µm), which provided a matrix for tissue sample perfusion. A 7-gauge needle was inserted into the tissue chamber from both sides of the syringe through the rubber stopper/plunger and covered at both the inlet and outlet with parafilm. On the rubber plunger side, the needle was connected to a 5-mL centrifuge tube that was used as the perfusion medium collector by the plastic tube (inner diameter, 1 mm; outer diameter, 2 mm). Twelve tissue chambers were simultaneously perfused in this system. The prepared chambers were immersed in a water bath at 37℃. The constant-flow pump with a 12-way channel was connected to the media reservoir by a plastic tube and each channel was connected to a tissue chamber by the appropriate plastic tube. Following the system setup, the KHB solution was introduced into the media reservoir and gassed with 95% O2 and 5% CO2 for the entire perfusion process. Before the experiment, the perfusion system was rinsed with a gassed KHB medium for at least 10 min. Subsequently, duodenum pieces were transferred into the tissue chamber and perfused with a KHB solution at 6 mL/h for 40 min as pre-perfusion, which was followed by perfusion with a KHB medium containing Trp, and [Ca2+]e or a CaSR antagonist for 120 min.
To investigate the response of the CCK and GIP secretion to Trp, the duodenum tissue was perfused with KHB as a basal solution (containing 1.8 mM [Ca2+]e) with 0 (the control group), 10, or 20 mM Trp at a final concentration for 120 min. The concentration of Trp used in the current study was based on the results of a mouse study conducted by Wang et al. [26]. To determine whether CaSR, PKC, and IP3R were activated by Trp, immunoblotting analysis was performed.
Duodenum tissue samples were collected at 30, 50, 70, and 90 min of perfusion with 0 and 20 mM Trp treatment. Ca2+ is the natural agonist of CaSR. In order to evaluate whether Ca2+ was involved in the secretion of gut hormones induced by Trp, three solutions were formulated: a basic KHB solution without Trp and Ca2+ (the control group); a KHB solution with 20 mM Trp; and a KHB solution with 20 mM Trp and 10 mM Ca2+. To evaluate the roles of CaSR and its downstream signaling pathway in the secretion of gut hormones induced by Trp, the duodenum tissue was exposed to a KHB solution containing a final concentration of 0 mM Trp (the control group), a KHB solution containing 20 mM Trp, or a KHB solution containing 20 mM Trp with the CaSR inhibitor NPS 2143 (MedChem Express, China) for 120 min. The duodenum tissue samples were obtained at 30, 50, 70, and 90 min after perfusion with 0 and 20 mM Trp treatment and every 10 min from 30 to 100 min after perfusion with 20 mM Trp and NPS 2143, and the protein levels of CaSR, PKC, and IP3R were assessed by immunoblotting analysis. In each treatment, a 2-mL perfusion medium was collected every 20 min during the entire perfusion process (120 min) and used to determine CCK and GIP concentrations by using enzyme-linked immunosorbent assay (ELISA) kits. At the end of perfusion, the duodenum tissue was harvested for the evaluation of CaSR mRNA expression by performing quantitative polymerase chain reaction (qPCR). Each experiment was performed by using samples from at least three independent pigs, and each treatment was replicated at least four times.
The concentrations of CCK and GIP in the perfusion supernatants were examined by using ELISA kits (CCK ELISA Kit [ANG-E31052P] and GIP ELISA Kit [ANG-E31000P]; Nanjing Angle Gene Biotechnology, China) according to the manufacturer's instructions. The antibodies used in the ELISA kits (anti-CCK [sc-21617] and anti-GIP [sc-23554]) were purchased from Santa Cruz Biotechnology, USA.
After perfusion, the RNA was isolated from the perfused duodenum tissue by using the RNAiso Plus reagent (9108; TaKaRa Technology, China) following the manufacturer's instructions. The extracted RNA was analyzed by using a Nanodrop spectrophotometer (Thermo Scientific, USA) to determine its concentration, purity, and integrity. RNA (1 µg) was treated with a gDNA eraser to eliminate genomic DNA and was reverse-transcribed to cDNA by using a PrimeScript RT reagent kit (RR047A; TaKaRa Technology) according to the manufacturer's recommendations. The qPCR was conducted according to the MIQE guidelines [3] and SYBR Premix Ex Taq (Tli RNaseH Plus, RR420; TaKaRa Technology). The amplification protocols included pre-denaturation of 95℃ for 30 sec, 40 cycles of denaturation at 95℃ for 15 sec, and annealing at 60℃ for 30 sec in a StepOnePlus Real-time PCR System (Life Technologies, USA). Specific amplifications of PCR products were verified by melting curve analysis. Glyceraldehyde phosphate dehydrogenase was used as the reference gene to normalize the CaSR expression, which was performed according to the 2−ΔΔCt method of StepOnePlus software (ver. 2.2.2; Life Technologies). All sample measurements were determined in triplicate. The sequences of the primers for the target and reference genes used in this study are presented in Table 1.
Extraction of total proteins from perfused duodenum tissue was performed by using tissue protein extraction reagent (78510; Thermo Scientific) in the presence of halt protease and phosphatase inhibitor cocktail (100×) (78440; Thermo Scientific). The concentration of total protein was determined by using a BCA protein assay kit (Enhanced; Beyotime, China). Samples (50 µg total protein) were resolved via sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoresis, the proteins were electrotransferred to a polyvinylidene fluoride membrane (Millipore, USA). After blocking by tris buffered saline (TBST, pH 7.5) with 5% BSA for 1 h at room temperature, the membrane was rinsed three times for 5 min each in TBST buffer and incubated at 4℃ overnight in TBST containing 3% BSA with primary antibody; mouse monoclonal anti-PKC (1:1,000) (ab23511; Abcam, UK), anti-CaSR (1:2,000) (MA1-934; Thermo Scientific), anti-β-actin (1:1,500) (sc-47778; Santa Cruz Biotechnology), rabbit polyclonal anti-IP3R (1:1,000) (ab5804; Abcam), respectively. Subsequently, the obtained blots were washed four times for 5 min each with TBST buffer and probed with either goat anti-mouse immunoglobulin G (IgG) (H + L) secondary antibody (1:5,000) (31160; Thermo Scientific) for PKC, CaSR, and β-actin or goat anti-rabbit IgG (H + L) secondary antibody (1:5,000) (31210; Thermo Scientific) for IP3R at room temperature for 1 h. Finally, the blots were rinsed five times for 5 min each and the steady-state levels of protein bands were visualized by applying SuperSignal West Dura Extended Duration Substrate (Thermo Scientific) and exposing them to an X-ray film. The immunoreactive protein bands were quantified by determining optical density with BandScan 5.0 software (Glyko, USA) [11].
The data are presented as mean ± SEM values. Statistical differences in gut hormone concentrations, the mRNA expression of CaSR, and protein levels of CaSR, PKC, and IP3R between groups were determined by using the one-way ANOVA procedure in IBM SPSS Statistics software (ver. 20.0; IBM, USA) followed by Tukey's multiple comparison tests. The p values < 0.05 were considered statistically significant, whereas p values < 0.01 were considered extremely significant.
Panels A to C in Fig. 2 shows the effect of Trp on the mRNA expression of CaSR and the concentrations of CCK and GIP in the perfusate. There was a significant elevation in the mRNA expression of CaSR with 10 and 20 mM Trp perfusion compared to that in the control group (p < 0.05; panel A in Fig. 2). In addition, higher concentrations of CCK were revealed with 10 and 20 mM Trp perfusions at 40 min and 100 to 120 min after perfusion initiation than that of the control group (p < 0.05; panel B in Fig. 2). Compared with the control group, a sharp elevation in the GIP secretion was observed after 20 min of perfusion with 20 mM Trp, and GIP secretion maintained a high level at 60 min of perfusion (p < 0.05; panel C in Fig. 2). At 120 min after perfusion, a significant increase of GIP secretion was also observed in the 20 mM Trp perfusion (p < 0.05; panel C in Fig. 2). However, no significant difference was observed in the release of GIP between the 10 mM Trp treatment and the control groups (p > 0.05; panel C in Fig. 2). Thus, the optimal concentration of Trp for perfusion was 20 mM, which was utilized in the following experiments.
The effect of [Ca2+]e on the CCK and GIP secretion response to Trp treatment is summarized in panels D to F in Fig. 2. The combination of 10 mM [Ca2+]e and 20 mM Trp exerted a significant promotion effect on the mRNA expression of CaSR (p < 0.05; panel D in Fig. 2). However, no significant difference was observed in the mRNA expression of CaSR between the perfusion of 20 mM Trp without [Ca2+]e and the control group (without Trp and [Ca2+]e) (p > 0.05; panel D in Fig. 2). The gut hormone secretion results showed that CCK and GIP secretions were greatly enhanced by Trp in the presence of 10 mM [Ca2+]e (p < 0.05; panels E and F in Fig. 2). However, the elevations in gut hormone secretion were not observed in the 20 mM Trp perfusion without [Ca2+]e (p > 0.05; panels E and F in Fig. 2). Therefore, these observations suggest that the Trp-induced secretions of CCK and GIP can be regulated by [Ca2+]e.
The results of the CaSR observations are shown in panels G to I in Fig. 2. The perfusion of 20 mM Trp without NPS 2143 significantly increased the expression of CaSR (p < 0.05; panel G in Fig. 2), whereas the presence of the CaSR antagonist NPS 2143 significantly downregulated the mRNA expression of CaSR (p < 0.05; panel G in Fig. 2). This gut hormone results showed that 20 mM Trp significantly promoted the release of CCK from 20 min to 120 min of perfusion (p < 0.05; panel H in Fig. 2) and promoted GIP release from 20 min to 100 min of perfusion (p < 0.05; panel I in Fig. 2). However, the promoting effects on CCK and GIP release by Trp induction disappeared in the presence of NPS 2143 (p > 0.05; panels H and I in Fig. 2). These findings suggest the involvement of CaSR in CCK and GIP secretion induced by Trp.
As shown in Figs. 3 and 4, 20 mM Trp increased the protein levels of CaSR and its downstream signal proteins PKC and IP3R after 30 min of perfusion compared with the levels in the control group (without Trp) (p < 0.05; Fig. 3). However, the addition of a specific CaSR inhibitor, NPS 2143, significantly suppressed the expression of CaSR induced by 20 mM Trp after 30 min to 100 min of perfusion (p < 0.05; Fig. 4). Furthermore, the NPS 2143 inhibition also significantly reduced the Trp-induced expressions of PKC and IP3R from 30 min to 100 min of perfusion (p < 0.05; Fig. 4). These observations indicate that CaSR and its signal proteins modulate the secretion of gut hormones induced by Trp.
Luminal gut sensing of L-amino acid levels is crucial in the regulation of diverse physiological processes, including the absorption of amino acids and the secretion of gut hormones [5]. In previous investigations, Trp was shown to evoke the secretion of satiety hormones by activating CaSR in humans and rodents [91524]. Relevant to these findings, Mace et al. [15] proposed a working model of CaSR in which the secretion of satiety hormones induced by amino acids was mediated by activating CaSR-mediated Ca2+ signaling pathways in rats. The results presented herein indicate for the first time that activation of CaSR is involved in the Trp-induced gut hormone responses to duodenal tissue perfusion in swine in vitro. This assertion is supported by four novel observations. First, 20 mM Trp stimulated an enhancement in the secretion of CCK and GIP as well as in the protein levels of CaSR, PKC, and IP3R in the presence of 1.8 mM [Ca2+]e. Second, the enhancement of the Trp-induced gut hormone response depended on [Ca2+]e. Third, inhibition of CaSR function with NPS 2143 impaired the elevation of CCK and GIP induced by Trp. Finally, the antagonism of Trp-driven CaSR activation with NPS 2143 reduced the protein levels of CaSR, PKC, and IP3R.
It has been shown that Ca2+ is a CaSR type 1 agonist, and Trp, a type 2 agonist of CaSR, requires [Ca2+]e to activate CaSR [21]. Studies on rodents have shown that both Phe and Trp promote the secretion of gut hormones via CaSR activation in the presence of [Ca2+]e at physiological concentrations [1415]. Our results showed that the secretion of CCK and GIP, as well as the mRNA expression of CaSR, were elevated by 20 mM Trp with 1.8 mM or 10 mM [Ca2+]e, and this response was reduced by the removal of [Ca2+]e. The results demonstrate that the function of Trp in inducing the responses in both CaSR and gut hormones is consistent with its function as a type 2 agonist of CaSR.
Therefore, we can conclude that Trp-activated CaSR works together with [Ca2+]e to regulate the secretion of gut hormones. It has been shown that CaSR is a functional sensor of amino acids [16], and it can mediate the secretion of gut hormones such as CCK, GIP, and PYY induced by certain amino acids in rodents [141526]. In our study, by using a perfusion system in vitro, we showed that the CaSR antagonist reduced the mRNA expression of CaSR and the secretions of CCK and GIP induced by Trp in the duodenum of pigs. These observations indicate that CaSR mediates the Trp-induced gut hormone secretion in swine duodenum, suggesting that CaSR has a crucial role in Trp-induced gut hormone release in swine. Therefore, the mechanism involves activation of CaSR by Trp, the subsequent generation of [Ca2+]i and the stimulation of the Ca2+-dependent signaling pathway, and the promotion of gut hormone exocytosis [1526].
The signaling pathway stimulated by CaSR for gut hormone secretion was proposed and described in recent studies of rodents [1527]. Trp was demonstrated to stimulate the CaSR, evoke phospholipase C (PLC)-mediated activation of IP3R and mobilization of [Ca2+]i stores, and result in the exocytosis of gut hormones in rodents [1527]. However, a CaSR-simulated signaling pathway for gut hormone release caused by amino acids in swine has not been reported. Our study, for the first time, observed that signaling pathways were activated by the stimulation of CaSR in the secretion of gut hormones induced by Trp in the duodenum of swine. The protein levels of CaSR, PKC, and IP3R were promptly elevated with perfusion of 20 mM Trp but were attenuated by the addition of CaSR inhibitor NPS 2143, suggesting that signaling pathways were involved in the stimulation of CaSR in the Trp-induced gut hormone secretion of pigs. Furthermore, the activation of CaSR by Trp may initiate PLC-mediated activation of IP3R, mobilization of [Ca2+]i stores, activate PKC-mediated signaling events, and induce gut hormone release [1015]. However, further investigation into the signaling pathway of CaSR activation via aromatic amino acids in the duodenum of swine is required to further elucidate the mechanisms by which aromatic amino acids activate CaSR and subsequent gut hormone secretion.
In summary, Trp perfusion of the duodenum tissue of swine can evoke CCK and GIP secretion in vitro by activating CaSR. The protein levels of CaSR and its signaling molecules PKC and IP3R were increased by Trp, but they decreased in the presence of a CaSR antagonist. Our study, for the first time, has revealed that Trp-induced CCK and GIP secretions can be mediated by the activation of CaSR and regulated by its downstream signal molecules PKC and IP3R in the duodenum of swine in vitro.
Figures and Tables
 | Fig. 1Schematic of the perfusion system (A) and the perfusion chamber (B). In (A): 1, oxygen bomb (mixture of 95% O2 and 5% CO2); 2, media reservoir; 3, peristaltic pump; 4, silicone tube; 5, perfusion chamber; 6, water bath (37℃); 7, collector. In (B): 1, nylon filter platform; 2, needle; 3, rubber stopper; 4, syringe; 5, rubber plunger. |
 | Fig. 2The mRNA expression of CaSR (A) and the secretion of gut hormones (B and C) regulated by different concentrations of tryptophan (Trp). The duodenal tissues were perfused with 0 (control), 10, and 20 mM Trp for 120 min. Tissues were collected at the end of perfusion. CaSR (A) mRNA expression was analyzed by quantitative polymerase chain reaction (qPCR) and glyceraldehyde phosphate dehydrogenase was used as the internal control. The concentrations of cholecystokinin (CCK, B) and glucose-dependent insulinotropic peptide (GIP, C) in the perfusate samples were detected every 20 min by using enzyme-linked immunosorbent assay kits. Values are expressed as mean ± SEM (n = 4). A statistical difference was determined by one-way ANOVA followed by Tukey's multiple comparison tests. Bars with the different letters show significant differences compared with the control group (A) (p < 0.05); * and δ (B and C); 20 mM Trp and 10 mM Trp treatments are significantly different from the control, respectively (p < 0.05). The influence of Ca2+ on the ability of Trp to induce the expression of CaSR (D) and the secretion of gut hormones (E and F). Tissue samples of duodenum were perfused with Krebs-Henseleit buffer containing 0 mM Trp without [Ca2+]e (control) and 20 mM Trp with or without 10 mM [Ca2+]e for 120 min. After perfusion, the expression of CaSR (D) was determined by qPCR. Perfusate samples were collected every 20 min to measure concentrations of CCK (E) and GIP (F). Values are expressed as mean ± SEM (n = 4). Statistical differences were determined by one-way ANOVA followed by Tukey's multiple comparison tests. Bars with the different letters showed the significant differences compared with the control group (D) (p < 0.05); γ (E and F); the combination of 20 mM Trp and 10 mM Ca2+ was significantly different from the control (p < 0.05). Effects of CaSR antagonist NPS 2143 on the expression of Trp-induced CaSR (G) and the secretion of gut hormones (H and I). Duodenal tissues were treated with 0 (control), 20, or 20 mM Trp and NPS 2143 (25 µM). At the end of perfusion (120 min), the duodenum tissues were collected to determine the mRNA expression of CaSR (G) by qPCR. The perfusate samples were obtained every 20 min to evaluate the concentration of CCK (H) and GIP (I). Values are expressed as mean ± SEM (n = 6). Statistical differences were determined by one-way ANOVA followed by Tukey's multiple comparison tests. Bars with the different letters showed the significant differences compared with the control group (G) (p < 0.05); χ (H and I); 20 mM Trp and the combination of 20 mM Trp and NPS 2143 treatments are significantly different from the control, respectively (p < 0.05). |
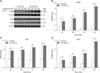 | Fig. 3Protein levels of calcium-sensing receptor (CaSR) and its signal molecules regulated by tryptophan (Trp). (A) The protein images of CaSR, protein kinase C (PKC), and inositol trisphosphate receptor (IP3R) at 0 and 20 mM Trp treatments. The optical densities of CaSR, PKC, and IP3R are shown in (B–D), respectively. Values are shown as mean ± SEM. **p < 0.01. |
 | Fig. 4The protein levels of calcium-sensing receptor (CaSR) and its signal molecules regulated by CaSR antagonist NPS 2143. (A) The protein images of CaSR, protein kinase C (PKC), and inositol trisphosphate receptor (IP3R) in the presence of 20 mM tryptophan and NPS 2143. The optical densities of CaSR, PKC, and IP3R are shown in (B–D), respectively. Values are shown as mean ± SEM. **p < 0.01. |
Acknowledgments
This work was supported by the National Basic Research Program of China (2013CB127301).
References
1. Badger TM. Perifusion of anterior pituitary cells: release of gonadotropins and somatotropins. Methods Enzymol. 1986; 124:79–90.
2. Bronk JR, Hastewell JG. The transport and metabolism of naturally occurring pyrimidine nucleosides by isolated rat jejunum. J Physiol. 1988; 395:349–361.

3. Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009; 55:611–622.

4. Conigrave AD, Brown EM. Taste receptors in the gastrointestinal tract. II. L-amino acid sensing by calcium-sensing receptors: implications for GI physiology. Am J Physiol Gastrointest Liver Physiol. 2006; 291:G753–G761.
5. Conigrave AD, Franks AH, Brown EM, Quinn SJ. L-amino acid sensing by the calcium-sensing receptor: a general mechanism for coupling protein and calcium metabolism? Eur J Clin Nutr. 2002; 56:1072–1080.

6. Cuber JC, Bernard C, Levenez F, Chayvialle JA. [Lipids, proteins and carbohydrates stimulate the secretion of intestinal cholecystokinin in the pig]. Reprod Nutr Dev. 1990; 30:267–275. French.
7. Hansen JR, Conn PM. Measurement of pulsatile hormone release from perifused pituitary cells immobilized on microcarriers. In : Conn PM, editor. Methods in Neurosciences. Vol. 2. Cell Culture. San Diego: Academic Press;1990. p. 181–194.
8. Hebert SC, Cheng S, Geibel J. Functions and roles of the extracellular Ca2+-sensing receptor in the gastrointestinal tract. Cell Calcium. 2004; 35:239–247.

9. Hira T, Nakajima S, Eto Y, Hara H. Calcium-sensing receptor mediates phenylalanine-induced cholecystokinin secretion in enteroendocrine STC-1 cells. FEBS J. 2008; 275:4620–4626.

10. Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003; 4:530–538.

11. Hou G, Xue L, Lu Z, Fan T, Tian F, Xue Y. An activated mTOR/p70S6K signaling pathway in esophageal squamous cell carcinoma cell lines and inhibition of the pathway by rapamycin and siRNA against mTOR. Cancer Lett. 2007; 253:236–248.

12. Karhunen LJ, Juvonen KR, Huotari A, Purhonen AK, Herzig KH. Effect of protein, fat, carbohydrate and fibre on gastrointestinal peptide release in humans. Regul Pept. 2008; 149:70–78.

13. Liddle RA, Green GM, Conrad CK, Williams JA. Proteins but not amino acids, carbohydrates, or fats stimulate cholecystokinin secretion in the rat. Am J Physiol. 1986; 251:G243–G248.

14. Liou AP, Sei Y, Zhao X, Feng J, Lu X, Thomas C, Pechhold S, Raybould HE, Wank SA. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells. Am J Physiol Gastrointest Liver Physiol. 2011; 300:G538–G546.

15. Mace OJ, Schindler M, Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J Physiol. 2012; 590:2917–2936.

16. Macleod RJ. CaSR function in the intestine: hormone secretion, electrolyte absorption and secretion, paracrine non-canonical Wnt signaling and colonic crypt cell proliferation. Best Pract Res Clin Endocrinol Metab. 2013; 27:385–402.

17. Miazza B, Palma R, Lachance JR, Chayvialle JA, Jonard PP, Modigliani R. Jejunal secretory effect of intraduodenal food in humans. A comparison of mixed nutrients, proteins, lipids, and carbohydrates. Gastroenterology. 1985; 88:1215–1222.

18. Moran-Ramos S, Tovar AR, Torres N. Diet: friend or foe of enteroendocrine cells--how it interacts with enteroendocrine cells. Adv Nutr. 2012; 3:8–20.

19. Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006; 444:854–859.

20. Neary MT, Batterham RL. Gut hormones: implications for the treatment of obesity. Pharmacol Ther. 2009; 124:44–56.

21. Nemeth EF, Steffey ME, Hammerland LG, Hung BC, Van Wagenen BC, DelMar EG, Balandrin MF. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci U S A. 1998; 95:4040–4045.

22. Rasoamanana R, Darcel N, Fromentin G, Tomé D. Nutrient sensing and signalling by the gut. Proc Nutr Soc. 2012; 71:446–455.

23. Steinert RE, Beglinger C. Nutrient sensing in the gut: interactions between chemosensory cells, visceral afferents and the secretion of satiation peptides. Physiol Behav. 2011; 105:62–70.

24. Steinert RE, Luscombe-Marsh ND, Little TJ, Standfield S, Otto B, Horowitz M, Feinle-Bisset C. Effects of intraduodenal infusion of L-tryptophan on ad libitum eating, antropyloroduodenal motility, glycemia, insulinemia, and gut peptide secretion in healthy men. J Clin Endocrinol Metab. 2014; 99:3275–3284.

25. Tan YF, Chen WH, Zou SX. The effect of β Casomorphin on piglets' stomach antrum under superfusion. J Nanjing Agric Univ. 2000; 23:72–75.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download