Abstract
Purpose
To analyze the complications after robot-assisted radical cystectomy (RARC) by use of a standardized reporting methodology by a single surgeon.
Materials and Methods
We prospectively reviewed a maintained institutional database of 52 patients who underwent RARC to manage bladder cancer and were followed up in 3 months by a single surgeon at Korea University Medical Center from 2007 through 2014. All complications within 90 days of surgery were defined and categorized into 5 grades according to the Clavien-Dindo classification. Logistic regression analysis was used to identify predictors of complications.
Results
Fifty percent of patients (26 of 52) experienced a complication of any grade <90 days after surgery, and 11 patients (21.2%) experienced a major complication. Complications were grouped in systems-based categories. Fifty complications occurred in 52 patients and hematologic complication (transfusion) was the most common (13 of 52). Wound dehiscence, anastomotic leakage, urinary tract obstruction, mechanical obstruction, and thromboembolism occurred as major complications. Mean estimated blood loss (EBL) was 247 mL and mean total operative time was 496 minutes. The mean number of lymph nodes harvested was 24.6, with 30.5 for extended dissection. EBL (over 300 mL), operative time, and method of urinary diversion were significant negative predictors of minor complications, whereas EBL (over 300 mL) was a significant negative predictor of major complications (p<0.05).
Open radical cystectomy with urinary diversion is considered the gold standard treatment for patients with invasive bladder cancer [1]. Despite improvements in surgical technique, technology, and perioperative care, cystectomy with urinary diversion remains a morbid operation with a substantial complication rate [2]. Recently, interest in laparoscopy and robot-assisted radical cystectomy (RARC) has increased with the goal of reducing procedure-related morbidity in the field of urology [3]. RARC has already superseded the use of pure laparoscopic radical cystectomy and is increasingly becoming an available option at major tertiary-care centers [4]. Therefore, RARC is representative of minimally invasive surgery for muscle-invasive transitional cell carcinoma of the bladder.
However, because adverse event reporting is highly variable and nonstandardized in the urologic literature, data on complications have mostly been reported by multiple surgeons. Also, most reports used only extracorporeal urinary diversion (ECUD) with a small skin incision to bring out the intestine following completion of robotic cystectomy [5]. Given that intracorporeal urinary diversion (ICUD) has only recently been attempted, studies regarding it have been rarely reported. In addition, it is difficult to analyze and interpret the data because the reporting system is not standardized. Also, most of the research is based on surgical outcomes that do not take into account the surgeons' technique. Therefore, reporting of complications on the basis of an established system is required.
We aimed to systemically analyze the complications after RARC performed by a single surgeon at a tertiary referral hospital including ICUD by use of a standardized reporting methodology.
We reviewed a prospectively maintained, Institutional Review Board-approved database of 52 patients who underwent RARC to manage bladder cancer by a single surgeon (S.H.K.) at Korea University Anam Hospital from 2007 through 2014. Complications and clinicopathologic data were collected by independent review of all inpatient charts, outpatient clinic records, nursing charts, and progression notes retrospectively. The data included age, sex, body mass index, American Society of Anesthesiologists score, history of neoadjuvant chemotherapy, pelvic radiotherapy, or surgery, clinical and pathologic stage, operative time, estimated blood loss (EBL), number of lymph nodes (LNs) removed, length of hospital stay, time to oral intake, and diversion type and method.
Patients were offered either orthotopic neobladder or ileal conduit, but ureterocutaneous diversion was performed in case of a single kidney. Urinary diversion was performed by both ECUD and ICUD (41 and 11 cases, respectively). Up to the 37th case, urinary diversion was performed extracorporeally; urinary diversion was then performed intracorporeally in the more recent cases.
All complications within 90 days of surgery were recorded and graded according to the established V-grade Clavien-Dindo classification. Clavien-Dindo grades I or II complications were categorized as minor complications, and grades III-V complications were considered major complications.
RARC was performed by a single surgeon at a single center. From 2007 to 2014, a total of 52 patients consecutively underwent RARC with pelvic lymph node dissection (PLND) by use of the da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA, USA). Of these patients, a consecutive series of 11 patients underwent RARC and a total ICUD from 2011. The surgical technique used for RARC with PLND including basic positioning and port placement was previously described [6,7,8]. The robot-assisted approach was implemented during cystectomy, during bilateral PLND, and for intracorporeal urethrovesical anastomotic stitches in cases of an orthotopic neobladder. In the case of ECUD with orthotopic neobladder, after excision of the bladder, the robot was undocked and urinary diversion was extracorporeally performed. Then the robot was redocked into the pelvis for urethroneovesical anastomosis with robotic assistance. In the case of ICUD, all procedures including urinary diversion were intracorporeally completed regardless of ileal conduit or orthotopic neobladder. PLND was initially conducted within the standard territory. Nonetheless, as the cases accumulated, the range extended to the inferior mesenteric artery or the aortic bifurcation including the common iliac, external iliac vessels, presacral, and obturator fossa. Nodal packets were separately harvested from each packet, and each resected nodal packet was individually extracted with the aid of a small organ bag to minimize metastasis via the trocar site.
Demographic, clinical, and pathologic variables were summarized by using descriptive statistics. The values for continuous variables are given as means (ranges). The statistical analysis was performed by using the Fisher exact test and chi-square test for categorical variables, which were specified as frequency (percentage). Univariable and multivariable logistic regression were used to predict the risk factors of complications. All statistical analyses were performed with PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA), and p-values below 0.05 were considered significant.
The clinical and pathological characteristics of the patients are summarized in Table 1. The demographic data and perioperative outcomes in patients who underwent ECUD or ICUD were compared as well. There were no significant differences in demographic data between the ECUD and ICUD groups. The mean age of the patients was 63.3 years (range, 39-79 years). Mean overall operative time was 496 minutes (range, 310-700 minutes) and mean EBL was 247 mL (range, 50-600 mL). Mean length of hospital stay was 17.3 days (range, 8-47 days). Thirteen patients (25%) underwent laparoscopic adhesiolysis owing to prior abdominal surgery before completion of the docking process. For urinary diversion, 27 patients (51.9%) had an ileal conduit, and 21 (40.4%) had an orthotopic neobladder. Four patients (7.7%) had an ureterocutaneostomy; two of them had a single kidney and the others underwent concomitant nephroureterectomy because ureteric frozen pathology was consistently positive during the operation. The mean number of LNs harvested was 24.6 (range, 4-71), with 30.5 (range, 8-71) in extended PLND.
Urinary diversion was performed both extracorporeally and intracorporeally. Up to the 37th case, it was performed extracorporeally. Urinary diversion was then performed intracorporeally in the more recent cases. In comparison with the ECUD group, mean overall operative time for the ICUD group was prolonged (464 minutes vs. 615 minutes, p<0.001), but mean EBL was lower (269 mL vs. 165 mL, p=0.003). In addition, cases of extended LND and the mean number of LNs harvested were greater in the ICUD group (p=0.01 and p=0.02, respectively).
Fifty percent of the patients (26 of 52) experienced at least one complication of any grade within 90 days of surgery, and 11 patients (21.2%) experienced a major complication (Table 2). The rate of complications was compared according to the diversion method as well. In the ECUD group, 56% of the patients (23 of 41) experienced at least one complication of any grade within 90 days of surgery. Twenty patients (48.8%) and nine patients (22%) each experienced a minor and a major complication. On the other hand, in the ICUD group, 27% of the patients (3 of 11) experienced at least one complication of any grade within 90 days of surgery; one patient (9.1%) and two patients (18.2%) each experienced a minor and a major complication. The incidence of minor complications for patients in the ECUD group was higher than for patients in the ICUD group (48.8% vs. 9.1%). There was a significant difference in the Fisher exact test (p=0.03) (Table 2).
Complications were grouped in systems-based categories (Table 3). Fifty complications occurred in 52 patients, and hematologic complication (transfusion) was the most common. Twenty-five percent of patients (n=13) required blood transfusion, and urinary tract infection (n=7) and fever (n=4) were the most common infectious complications. Wound dehiscence, anastomotic leakage, urinary tract obstruction, mechanical obstruction, and thromboembolism occurred as major complications in 11 patients. One patient developed mechanical obstruction. Therefore, the patient underwent an additional colorectal operation for repair. Five patients needed a wound revision owing to wound dehiscence. All of them underwent RARC with the ECUD method. Among them, three patients underwent wound closure, which was performed under general anesthesia and thus was categorized as a major complication. Two patients underwent simple suturing under local anesthesia as a minor complication. Anastomotic leakage occurred in four patients; three of them required catheterization for drainage of ureteral anastomotic leakage, and one patient underwent an operation for repair of urethral anastomotic leakage. Urinary tract obstruction occurred in three patients. One patient had to undergo transuretero-ureterostomy, another patient needed to have ureterocutaneostomy stoma revision, and the other only underwent temporary catheterization. Vascular thrombo-embolitic events occurred in two patients. Atherosclerosis obliterans of the left toe occurred in one patient; therefore, he needed a procedure for stent insertion. Medication was enough for the other patient who developed a deep vein thrombosis.
Regarding predictors of major complications, in the univariable and multivariable analyses, EBL (over 300 mL) was significant in predicting a complication (Table 4). Concerning predictors of minor complications, EBL (over 300 mL), operative time, and method of urinary diversion (extra- vs. intracorporeal) were significant negative predictors in the multivariable analysis, whereas EBL (over 300 mL) and method of urinary diversion were significant negative predictors in the univariable analysis. EBL (over 300 mL) was a significant negative predictor of total complications in the univariable analysis, and EBL (over 300 mL) and operative time were significant negative predictors of total complications in the multivariable analysis. No patients died within 90 days.
Standardized reporting of postoperative complications is vital for treatment planning, clinical trial design, assessing innovative surgical techniques, and informed patient counseling [9]. However, adverse event reporting is highly variable and nonstandardized in the urologic literature, and the complication data have mostly been reported by multiple surgeons. Most studies evaluating the morbidity of radical cystectomy do not use a formal grading system, account for comorbidities, or clearly define various complications [9]. Consequently, it is difficult to interpret the data owing to the nonstandardized reports and inconsistent surgical outcomes by multiple surgeons with different surgical techniques.
We aimed to analyze the complications after RARC performed by a single surgeon at a single center by use of a standardized reporting methodology. At our institution, 50% of patients (26 of 52) experienced at least one complication of any grade within 90 days of surgery, and 11 patients (21.2%) experienced major complications (Table 2). These results show that RARC has fewer complications than in recently published open series reported by use of a similar methodology. Shabsigh et al. [10] used a standardized reporting methodology in a series of 1,142 open radical cystectomy patients. Within 90 days postoperatively, 64% of patients experienced a complication, and the major complication rate was 13%. Recently, smaller series reviewing initial complication rates of RARC have reported complication rates ranging from 34% to 63% [3,11,12]. Pruthi et al. [13] reported an overall complication rate of 36% among 100 consecutive RARC cases but included complications that occurred within only 30 days of cystectomy. Ng et al. [14] reported a 48% complication rate within 90 days of RARC by use of standardized reporting methods. More recently, in a series of 156 RARC patients, Hayn et al. [11] identified 52% of patients having experienced a complication within 90 days of surgery, and the major complication rate was 24%. Yuh et al. [2] reported that the 90-day complication rate was 80% by use of the modified Clavien-Dindo system, and the major complication rate was 35%. The 50% complication rate and 21.2% major complication rate in our study are comparable to those of contemporary robot series.
Fif ty complications occurred in 52 patients, and transfusion was the most common complication (n=13). Transfusion rates f or contemporary open radical cystectomy series have ranged from 38% to 66% [10,15]. For robot series, Yuh et al. [2] and Hayn et al. [11] reported rates of 43.9% and 16%, respectively. In our study, the transfusion rate was 25%. The results suggest that the robotic surgery helps to reduce blood loss and the need for blood transfusion.
The mean operative time of 496 minutes is longer than in many open series and other RARC series. Hayn et al. [11] reported a median operative time of 378 minutes and Yuh et al. [2] reported 432 minutes. The time difference may be explained by the urinary diversion type and learning curve. Hayn et al. [11] reported that 7% of patients (11 of 156) underwent orthotopic neobladder formation, and Yuh et al. [2] reported that 44% of patients (86 of 196) underwent orthotopic neobladder formation [2,11]. In our study, 21 patients (40.4%) underwent orthotopic neobladder, and 11 patients (21%) underwent ICUD in the RARC group. We also need to consider the associated learning curve.
In open RC, Shabsigh et al. [10] reported that age, prior abdominal surgery, and EBL were significantly associated with experiencing a grade III or higher complication. Similarly, Yuh eh al. [2] reported that comorbidity, preoperative hematocrit, and orthotopic diversion were significantly associated with experiencing major complications. In our study, as predictors of minor complications, EBL (over 300 mL), operative time, and method of urinary diversion were significant in the multivariable analysis. EBL (over 300 mL) and operative time were signif icant negative predictors of total complications in the multivariable analysis. EBL (over 300 mL) was a significant predictor of major complications in the univariable and multivariable analyses.
Urinary diversion performed by using a total ICUD method can have additional benefits, including less incisional pain, decreased bowel exposure and desiccation, and the potential for decreased fluid imbalances [16]. Because ICUD has only recently been attempted, studies related to it are scarce. Hence, determining whether to create a complete ICUD versus performing the reconstruction extracorporeally is still a controversial and critical issue [8]. Pruthi et al. [16] compared 32 cases of RARC with ICUD (n=12) or ECUD (n=20). Although the operation time was longer in ICUD patients (5.3 hours) than in ECUD patients (4.2 hours), the complication rates within 30 days were similar between the two groups (42% vs. 40%). Guru et al. [17] reported a comparison between 13 ICUD and 13 ECUD procedures, with four complications in the ICUD group and five in the ECUD group in a 3-month follow-up. In our study, we also compared the rates of complications between the urinary diversion methods. We found a difference in operative time between the ECUD group (464 minutes) and the ICUD group (615 minutes). As a result, the incidence of minor complications was higher for patients in the ECUD group than for patients in the ICUD group (48.8.0% vs. 9.1%). There were significant differences in the Fisher exact test (p=0.03) (Table 2). Yet, there was no significant difference in major complications. As a predictor of minor complications, ECUD was a significant factor in the univariable and multivariable analyses (p<0.05). The reason that ECUD is regarded as a risk factor is due to the fact that ICUD, which was performed later in the series, had fewer complications because the learning curve had already been achieved. Also, the number of patients who underwent ICUD may have been too few to achieve statistical significance. Recently, a study of an international robotic cystectomy consortium with 935 patients reported that the 90-day complication rate was not significantly different between the groups, but a trend favoring ICUD over ECUD was noted (41% vs. 49%, p=0.05) [18]. As mentioned before, ICUD is considered a leader in the minimally invasive trend, although it has vulnerability. For the practical application of ICUD, more global data with respect to long-term outcomes and a larger series of cases are required.
This study had limitations. It was a retrospective and nonrandomized study and may have contained selection bias. The sample size was relatively small, which limits the statistical power of the analysis as well. Because the learning curve varies according to the surgeon's technical characteristics, supporting data from other surgeons with similar experience levels will be needed to identify the advantages of RARC in the early learning period.
The present study showed that the complication rate reported by use of a standardized methodology after robotic radical cystectomy is still considerable, although comparable to those of contemporary robot series. EBL (over 300 mL), operative time, and diversion methods were predictors of complications after RARC. ICUD has a lower risk of postoperative complications. Larger, randomized studies are required to compare complications between open radical cystectomy and robotic radical cystectomy in order to define the role of robotics in radical cystectomy.
Figures and Tables
Table 1
Patient characteristics and perioperative outcomes

Table 2
Complication summary

Table 3
Complication summary in systems-based categories
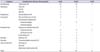
Table 4
Univariable and multivariable logistic regression analysis of complications
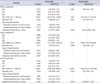
OR, odds ratio; CI, confidence interval; ASA, American Society of Anesthesiologists classification; EBL, estimated blood loss; ECUD, extracorporeal urinary diversion; ICUD, intracorporeal urinary diversion.
Receipt of neoadjuvant chemotherapy, prior abdominal surgery, and prior pelvic radiotherapy were also tested in the univariable and multivariable models, with negative statistical results.
ACKNOWLEDGMENTS
This study was supported by a research grant from the Korea University College of Medicine (Seoul, Korea).
References
1. Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001; 19:666–675.
2. Yuh BE, Nazmy M, Ruel NH, Jankowski JT, Menchaca AR, Torrey RR, et al. Standardized analysis of frequency and severity of complications after robot-assisted radical cystectomy. Eur Urol. 2012; 62:806–813.
3. Sung HH, Ahn JS, Seo SI, Jeon SS, Choi HY, Lee HM, et al. A comparison of early complications between open and robot-assisted radical cystectomy. J Endourol. 2012; 26:670–675.
4. Hemal AK. Role of robot-assisted surgery for bladder cancer. Curr Opin Urol. 2009; 19:69–75.
5. Woods ME, Wiklund P, Castle EP. Robot-assisted radical cystectomy: recent advances and review of the literature. Curr Opin Urol. 2010; 20:125–129.
6. Kang SG, Kang SH, Lee YG, Rha KH, Jeong BC, Ko YH, et al. Robot-assisted radical cystectomy and pelvic lymph node dissection: a multi-institutional study from Korea. J Endourol. 2010; 24:1435–1440.
7. Kang SC, Kang SG, Choi H, Ko YH, Lee JG, Kim JJ, et al. The feasibility of robot-assisted laparoscopic radical cystectomy with pelvic lymphadenectomy: from the viewpoint of extended pelvic lymphadenectomy. Korean J Urol. 2009; 50:870–878.
8. Kang SG, Ko YH, Jang HA, Kim J, Kim SH, Cheon J, et al. Initial experience of robot-assisted radical cystectomy with total intracorporeal urinary diversion: comparison with extracorporeal method. J Laparoendosc Adv Surg Tech A. 2012; 22:456–462.
9. Donat SM. Standards for surgical complication reporting in urologic oncology: time for a change. Urology. 2007; 69:221–225.
10. Shabsigh A, Korets R, Vora KC, Brooks CM, Cronin AM, Savage C, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009; 55:164–174.
11. Hayn MH, Hellenthal NJ, Hussain A, Stegemann AP, Guru KA. Defining morbidity of robot-assisted radical cystectomy using a standardized reporting methodology. Eur Urol. 2011; 59:213–218.
12. Khan MS, Elhage O, Challacombe B, Rimington P, Murphy D, Dasgupta P. Analysis of early complications of robotic-assisted radical cystectomy using a standardized reporting system. Urology. 2011; 77:357–362.
13. Pruthi RS, Nielsen ME, Nix J, Smith A, Schultz H, Wallen EM. Robotic radical cystectomy for bladder cancer: surgical and pathological outcomes in 100 consecutive cases. J Urol. 2010; 183:510–514.
14. Ng CK, Kauffman EC, Lee MM, Otto BJ, Portnoff A, Ehrlich JR, et al. A comparison of postoperative complications in open versus robotic cystectomy. Eur Urol. 2010; 57:274–281.
15. Lowrance WT, Rumohr JA, Chang SS, Clark PE, Smith JA Jr, Cookson MS. Contemporary open radical cystectomy: analysis of perioperative outcomes. J Urol. 2008; 179:1313–1318.
16. Pruthi RS, Nix J, McRackan D, Hickerson A, Nielsen ME, Raynor M, et al. Robotic-assisted laparoscopic intracorporeal urinary diversion. Eur Urol. 2010; 57:1013–1021.
17. Guru K, Seixas-Mikelus SA, Hussain A, Blumenfeld AJ, Nyquist J, Chandrasekhar R, et al. Robot-assisted intracorporeal ileal conduit: Marionette technique and initial experience at Roswell Park Cancer Institute. Urology. 2010; 76:866–871.
18. Ahmed K, Khan SA, Hayn MH, Agarwal PK, Badani KK, Balbay MD, et al. Analysis of intracorporeal compared with extracorporeal urinary diversion after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol. 2014; 65:340–347.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download