Abstract
Quality of life is adversely affected by pelvic organ prolapse, the prevalence of which is increasing because of the persistently growing older population. Today, the tension-free vaginal mesh kit has grown in popularity owing to its comparable cure rate to traditional reconstructive surgery and the feasibility of an early return to normal life. However, significant debate remains over the long-term cure rate and the safety of tension-free vaginal mesh in the United States. The U.S. Food and Drug Administration recommends obtaining informed consent about the safety and cure rate when the patient chooses surgery using the tension-free vaginal mesh kit or meshes before surgery. The goal of surgery for pelvic organ prolapse is the restoration of anatomic defects. This review article provides an overview of basic surgical techniques and the results, advantages, and disadvantages of surgery for pelvic organ prolapse.
Pelvic organ prolapse (POP) is defined as the descent of one or more of the following: anterior vaginal wall, posterior vaginal wall, apex of the vagina (cervix to uterus), or vault (cuff) after hysterectomy. POP affects almost half of all women over 50 years of age, with a lifetime prevalence of 30% to 50% [1]. Women with a normal life expectancy will have an 11% to 12% chance of undergoing at least one operation for prolapse or incontinence, with a reoperation rate of 29% by the age of 79 years [2].
The etiologies of POP include aging, pregnancy, delivery, and previous pelvic surgery, in addition to combined high-risk factors that increase intra-abdominal pressure, such as chronic pulmonary disease, constipation, obesity, and heavy manual labor. The etiologies of POP weaken the pelvic floor muscles and ligaments, which support the bladder, urethra, uterus, and rectum, which can lead to detachment from the ligaments or pelvic bone where the muscles were attached.
The management of POP includes nonsurgical and surgical management. Most pelvic surgeons favor surgical treatment. However, conservative management can be applied to patients with a low Pelvic Organ Prolapse Quantification system (POP-Q) stage, those who decline surgery, and elderly patients with multiple comorbidities. This article reviews the basic principles, cure rates, advantages, disadvantages, and complications of each procedure.
Nonsurgical treatments consist of conservative management and the use of mechanical devices.
Behavioral modification and pelvic floor muscle exercise (PFME) are the mainstay of conservative treatment. The purpose of conservative treatment is the reduction of symptoms, the prevention of worsening POP, increased support of the pelvic floor musculature, and avoiding or delaying surgery [3]. Behavioral modification includes the reduction of high-risk factors that provoke chronic increases in abdominal pressure, such as constipation, obesity, chronic cough, and cigarette smoking.
PFME was introduced by Kegel [4] for the treatment of postpartum sexual dysfunction and stress urinary incontinence in 1948. To improve muscle strength, the contraction should be sustained for 2 to 10 seconds [5]. PFME should be performed regularly, in two to three sessions per day. Each session consists of 10 sustained contractions within 20 minutes. PFME is suitable for mild to moderate POP but not for high-grade POP (POP-Q stage III and IV).
Pessaries have been used in POP since the era of Hippocrates [6]. To prevent allergic and toxic reactions, most pessaries are made of medical-grade silicone. The indications for pessary use are inoperable medical status owing to medical comorbidity or refusal of surgery. The relative contraindications are a wide vaginal outlet, short vagina, desire for surgery, and an inability to manage the pessary by the patient herself (insertion and removal of the pessary periodically) [7]. There are many types of pessaries that vary according to the company and their indication. Two categories of pessaries are used. One category includes pessaries with a supportive function, such as a ring pessary for stages I and II POP. The other category includes pessaries of the space-filling type, such as Cube and Inflato ball pessaries for stages III and IV POP [8].
In one study, pessaries were fitted successfully to 74% of 110 women who selected pessary management. Among the 62 women who used a pessary for more than 1 month, 66% were still using it after 12 months [9]. The complications of pessary use include vaginal erosion, pelvic pain, vaginal discharge, severe stress urinary incontinence, de novo voiding difficulty, and de novo defecation difficulty [9,10].
There are typical patterns of surgery: (1) restorative surgery using the patient's endogenous supportive tissue, (2) compensatory surgery using synthetic meshes or biological graft materials, and (3) obliterative surgery, which partially or totally closes the vagina. The purpose of surgical management of POP is the correction of anatomic defects. Surgical routes are through the vagina or abdomen and include laparotomy and the use of a laparoscope or robotic system (Table 1).
Anterior vaginal wall prolapse is defined as the descent of the anterior vagina such that the urethro-vesical junction (a point 3-cm proximal to the external urethral meatus) or any anterior point proximal to this is less than 3 cm above the plane of the hymen. Cystocele is classified as a paravaginal defect (lateral, displacement), midline defect (central, distention), or transverse defect (apical) when the pubocervical fascia separates from the vaginal cuff or uterosacral ligaments or a combination of these defects.
Lateral cystocele is caused by the detachment of the pubocervical fascia and pubourethral ligaments from the arcus tendineus fasciae pelvis (ATFP). According to the mechanism of lateral cystocele, the purpose of the proposed operation is the correction of the relaxed or detached pubocervical and pubourethral ligaments.
Transvaginal paravaginal repair: After the perforation of endopelvic fascia, palpation is applied to identify the ATFP; because the surgical field is so deep, light supported retractors are useful. With the bladder retracted medially, five to seven interrupted nonabsorbable sutures are placed at 1-cm intervals bilaterally. In recent times, trocar-guided transvaginal mesh insertion has generally been performed to reinforce the defect, and diverse mesh materials have been used (acellular collagen biomesh, etc.) [11,12]. After the sutures are tied, cystoscopy is performed to identify the efflux from ureteral orifices and bladder injury. The success rates of transvaginal paravaginal repair have been reported to range from 82.5% to 98% [11,12,13].
Retropubic paravaginal repair: This approach is recommended in the case of a Burch operation or total abdominal hysterectomy. The detached pubocervical fascia is sutured with nonabsorbable sutures to the ATFP with an interval of 1 cm from the ischial spine to the pubic bone. The reported success rate of this procedure is from 85% to 98% [14,15]. Recently, most retropubic paravaginal repair has been performed by use of laparoscopic technique, and the cure rate of this procedure is comparable to that of open surgery [16]. Complications include intraoperative hemorrhage, lower extremity neuropathy, vaginal abscess, and ureteral obstruction.
The cause of central cystocele is the failure of support by the pubocervical fascia at the midline of the anterior vaginal wall. The aim of surgical repair is the approximation of relaxed or separated pubocervical fascia.
Transvaginal anterior colporrhaphy: This procedure was developed by Kelly in 1913. Many variable modifications have been described. However, the basic principle is an approximation of the detached pubocervical fascia with 2-0 delayed absorbable sutures interruptedly. The cure rate has been reported to be up to 97% [17]. The most recent report suggests that the recurrence rates for anterior colporrhaphy approach 70% [18].
In cases of a severely weakened pubocervical fascia, synthetic meshes or biological grafts are placed under the repaired pubocervical fascia [19]. Sand and associates compared the result of anterior colporrhaphy and anterior colporrhaphy with free polyglactin mesh inlay below the plicating pubocervical fascia. After 1 year of follow-up, the randomized female patients who received the polyglactin mesh had a failure rate of 25%, compared with the 43% failure rate in those women who received anterior colporrhaphy only [20]. Natale et al. [21] compared the efficacy of polypropylene mesh and porcine dermis in the surgical treatment of recurrent cystocele. The objective cure was 71.9% for polypropylene mesh and 56.4% for porcine dermis (p=0.06). According to a recent report from the New England Journal of Medicine, a transvaginal mesh repair group showed a higher cure rate (60.8%, 107/176 patients) than did a traditional anterior colporrhaphy group (34.5%, 60/174 patients) at 1 year after the surgery, although the mesh repair group showed a higher bladder perforation rate and a higher rate of new stress urinary incontinence than did the anterior colporrhaphy group (3.5% vs 0.5% and 12.3% vs 6.3%, respectively) [22]. This procedure is not recommended for lateral defects or stress urinary incontinence.
Intraoperative complications of transvaginal anterior colporrhaphy are uncommon. Excessive blood loss and hematoma, trauma to the bladder and urethra, ureteral damage and obstruction, urinary tract infections, and voiding difficulty may occur.
Intra-abdominal anterior repair: In the case of central cystocele only, this procedure is not recommended. However, in the case of mild high central cystocele during an abdominal surgery, such as hysterectomy, intra-abdominal anterior repair can be undertaken. After dissection between the bladder and vagina, wedge resection of the redundant vaginal wall is performed, and interrupted or running sutures can be placed. Lovatsis and Drutz [23] reported a success rate for grade 1 cystocele of 89%. There was a considerable deterioration over time with failure rates of 30% after 2 years and 61% after 5 years.
Most cystoceles include the combination of lateral and central defects. The purpose of surgery is the correction of associated defects.
Transvaginal anterior repair and paravaginal repair: Anterior colporrhaphy with paravaginal repair can be performed as previously mentioned. Nonetheless, difficulties exist in performing paravaginal repair after anterior colporrhaphy. Different vector forces are applied on the pubocervical fascia; one is directed medially, and the other is directed to the outside. This differential application of vector forces is one reason the manufacturer developed synthetic mesh or biological graft material kits. Currently, despite arguments against synthetic mesh, some urologists and gynecologists continue to use this procedure because of its additional advantages and convenience [24]. Rodriguez et al. [25] reported an 84% cure rate with stage 3 (grade IV) cystocele.
Transabdominal anterior repair and paravaginal repair: The transabdominal approach is not recommended for patients with lateral and central cystocele only without comorbidities. Additionally, the success rate of abdominal anterior repair may be limited because of the inability to approximate the pubocervical fascia [23].
For women with apparent or occult stress urinary incontinence in association with advanced prolapse, placement of a midurethral mesh sling or Burch procedure results in higher continence rates than suburethral plication or paravaginal repair alone.
Posterior vaginal wall prolapse is defined as any descent of the posterior vaginal wall so that a midline point on the posterior vaginal wall 3 cm above the level of the hymen or any posterior point proximal to this point is less than 3 cm above the plane of the hymen. Posterior vaginal wall relaxation is caused by attenuation or site-specific tearing of the rectovaginal fascia, which results in herniation of the rectum and small intestine in approximately 40% of asymptomatic parous women [26].
Rectoceles are subdivided by location and anatomical defects as low, midvaginal, or high rectocele. High rectocele is the result of defects in the cardinal/uterosacral ligament complex. Midvaginal rectocele is caused by the weakening or detachment of the rectovaginal fascia from the ATFP. Separation of the perineal body at the level of the rectovaginal fascia results in perineal descent or a low rectocele. The other classifications of rectocele are according to the tearing portion of rectovaginal fascia: lateral defect, central defect, apical defect, and perineal defect [27].
Nonsurgical treatments consist of proper bowel training, following an active lifestyle, and eating an appropriate amount of dietary fiber to aid with constipation. Other non-surgical treatments include hormonal replacement therapy for menopausal women and vaginal pessary use. Indications for surgery include having symptoms that respond well to surgery, including pelvic pressure, vaginal bulge and vaginal splinting for defecation, anatomic defects, or undergoing another POP surgery.
This procedure involves the plication of the pubococcygeus muscles across the anterior rectum as well as perineal body reconstruction. This procedure has complications such as severe postoperative pain, dyspareunia, and vaginal stricture [28,29]. To prevent these complications, the repaired opening should accommodate two to three finger breadths, taking into account the fact that the levator ani and perineal muscles are relaxed from general anesthesia and may further constrict postoperatively and undergo postmenopausal atrophy. The modified technique includes site-specific repair and midline fascial plication without levator plication. The success rate of traditional posterior repair is reported to be from 78% to 97% [30,31], whereas that of midline fascial plication is from 79% to 92.1% [32,33,34].
After separation between the vaginal epithelium and fibromuscular layer, insertion of a finger in the rectum makes the differentiation of site-specific defects possible. Interrupted or running sutures with delayed absorbable sutures are utilized. Next, plicating fascia is used to cover the area with interrupted 2-0 delayed absorbable sutures. Afterwards, evaluation of the levator hiatus is performed and, in turn, narrowing of the levator hiatus with interrupted 1-0 delayed absorbable sutures is completed. If needed, perineal body reconstruction can be performed. The success rate of site-specific repair ranges from 59% to 100% [36,37]. The de novo constipation rate is from 3% to 4% [38,39], and the de novo dyspareunia rate is from 0% to 8% [34,37,38,39,40].
Graft material may be used in both the traditional posterior colporrhaphy and the defect-specific technique in an attempt to strengthen the repair. Sand reported no difference in recurrence after posterior repair with or without mesh reinforcement [20]. Paraiso et al. [41] reported that the addition of a porcine-derived graft does not improve anatomic outcomes. However, in the case of high rectocele repair, there are defects of the rectovaginal and pubocervical fascia proximally; this potential space for enterocele can be closed with synthetic meshes or biological graft materials.
Prolapse of the apical segment of the vagina is defined as any descent of the vaginal cuff scar (after hysterectomy) or cervix below a point that is 2 cm shorter than the total vaginal length above the plane of the hymen. Transvaginal and abdominal approaches are used according to patient status and comorbidity.
Sacrospinous ligament fixation: Sacrospinous ligament fixation was widely used after the report by Randall and Nichols [42] in 1971. The main indication for sacrospinous ligament fixation is to correct total procidentia or post-hysterectomy vaginal vault prolapse with an associated weak cardinal uterosacral ligament complex and to correct posthysterectomy enterocele. The contraindication for the procedure is a short vagina.
The principle of this procedure is the fixation of the vaginal vault to the sacrospinous ligament with nonabsorbable sutures. The fixation site is typically the right sacrospinous ligament. However, bilateral fixation is performed in patients with recurrent vault prolapse and with the goal of restoring a vaginal axis and sexual life. The routes of entry to the sacrospinous ligament may be posterior and anterior. Usually, a unilateral, right-sided, posterior approach is preferred.
Meuman et al. [43] reported apical success rates of 95.5%. Hefni and El-Toukhy [44] have reported success rates of approximately 96% with follow-up times ranging from 24 to 84 months. The most common causes of failure are poor approximation of the vault to the ligament, i.e., the suture bridge [45], and postoperative infection [46].
This procedure has advantages, including success rates comparable to abdominal procedures, the ability to repair concomitant pelvic floor defects, the absence of laparotomy, shorter hospital stays, and the preservation of vaginal length and function. The most common problem after this procedure is the high rate of postoperative cystocele, which approaches 20% to 33% [47,48], resulting from the deviation of the vaginal axis. Recurrent cystoceles have been reported in 6% to 92% of patients. Other disadvantages include difficulty in exposing the ligament, the potential need for excessive tensioning during tying, injury risk to the pudendal or inferior gluteal vessels and sciatic or pudendal nerve, alterations in the vaginal axis, and vaginal narrowing. Thomson et al have reported that by placing the sutures through the sacrospinous ligament 2.5 cm more medially from the ischial spine along the superior border of the ligament and not through the full thickness of the ligament, the risk of complications is minimal [49].
Uterosacral vaginal vault suspension: Uterosacral vaginal vault suspension can be performed intra-abdominally after hysterectomy. This procedure is a fixation of both lateral sides of the vaginal vault to the uterosacral ligament bilaterally. The other route can be through the vagina. After vaginal hysterectomy, both sides of the vaginal vault are fixed to the uterosacral ligament. If this procedure is applied for vaginal vault prolapse, the uterosacral ligament must be identified by palpation. During the procedure, attention to the ureter is needed because the ureter runs through the lateral side of the uterosacral ligament. Thus, after the surgery, cystoscopy must be performed to confirm ureteral efflux and bladder injury. The success rates are 95% to 98% within the first few years [50,51]. In recent times, Cosma et al. [52] compared synthetic mesh and native ligament vaginal vault suspension in patients with stages III-IV uterovaginal apical prolapse after hysterectomy. After 56.2 and 57.7 months of mean follow-up for mesh repair and native ligament repair, respectively, the native ligament repair group showed a higher cure rate without complications compared with the mesh repair group (90.2% vs. 100%). They recommended that native tissue repair be considered with priority for vaginal hysterectomy, and that mesh repair be considered for recurrent vault prolapse and for selective cases such as complete uterovaginal eversion.
Iliococcygeus suspension: The principle of iliococcygeus suspension is the fixation of the vaginal vault to the fascia of the iliococcygeus muscle bilaterally. This procedure can be performed in cases where the uterosacral ligament is not identified or is of insufficient length to attach to the vaginal vault. This procedure has the proposed advantages of decreasing neurovascular injury or postoperative cystocele. Meeks et al. [53] reported an anatomic success rate of 96% in 110 patients. Maher and associates reported a 53% cure rate in 50 patients, and 19% of the patients experienced buttock pain [54]. Medina et al. [55] reported that iliococcygeus suspension does not shorten the vaginal length, whereas sacrospinous ligament suspension does. This is one of the advantages of iliococcygeus suspension, because maintaining vaginal length is an important factor for the recurrence of POP and is also essential for coital function.
Posterior intravaginal slingoplasty: Petros [56] developed the posterior intravaginal slingoplasty procedure and reported the results of 75 patients with vaginal vault prolapse. Surgical techniques include level I repair, which aims to insert a tape in the position of the uterosacral ligament by use of a tunneler. Level II repair aims to approximate the rectovaginal fascia towards the midline, and level III includes repairing the perineal body. Farnsworth reported a 91% cure rate in 93 patients. The median follow-up was 12 months. Many commercial kits have been introduced in the market [57]. When comparing slingoplasty with sacrocolpopexy, the same efficacy was reported for both anatomical and symptomatic improvement [58].
Lane first proposed abdominal sacrocolpopexy in 1962 [59]. The basic concept of the procedure is support of the vagina with a suspensory bridge connected to the anterior longitudinal ligament of the sacrum. This procedure is indicated in patients with failed previous vaginal repair, in patients with isolated apical prolapse or enterocele, and in younger women with an active sexual life.
In this procedure, the vaginal vault is fixed to the anterior longitudinal ligament of the sacral promontory with biological graft materials or synthetic mesh, preferably macroporous polypropylene mesh. The procedure is more durable because of less paravaginal scarring and denervation and the fixation of the entire vaginal apical area by a permanent mesh to the stable ligament. The procedure maintains a maximal vaginal length and near-normal axis. If enterocele is present, the peritoneal cul-de-sac is closed with the Halban or Moschcowitz procedure [60].
Nygaard reported that anatomic success rates range from 78% to 100% and that the rates of satisfaction and complete relief of symptoms range from 85% to 100% [61]. The reported success rates were superior to vaginal sacrospinous ligament fixation in terms of the rate of recurrence (3 of 84 vs. 13 of 85), the number of women failing to improve to stage 2 or better (3 of 52 vs. 13 of 66), and less postoperative dyspareunia (7 of 45 vs. 22 of 61) [62].
Abdominal sacrocolpopexy is considered the gold standard procedure for the surgical correction of vaginal vault prolapse based on numerous level 1 studies reporting its efficacy and long-term durability [63]. The complications of this procedure include vaginal erosion of the graft material or suture (3.4%), intraoperative hemorrhage (4.4%), postoperative ileus (3.6%), and small bowel obstruction (1.1%) [61]. Retroperitonealization of the mesh is needed to prevent bowel complications.
Enterocele is prolapse of the peritoneal cul-de-sac (the pouch of Douglas) with or without the bowel and is usually caused by apical or high posterior compartment defects. Complete vaginal vault prolapse is accompanied by enterocele in 75% of patients [64]. The surgery for enterocele is obliteration of the pouch of Douglas.
During the vaginal correction of the apical compartment, enterocele can be corrected. After identifying the sac of enterocele, the sac is opened, a finger is inserted into the sac, and the intestinal content is displaced back into the abdomen. One or two purse-string sutures are placed around the neck of the enterocele sac. Three sets of 0 synthetic absorbable sutures should be placed between the anterior rectal wall, the stump of the enterocele sac, and the uterosacral ligaments [65]. The cure rate is reported to be 67% [63].
During intra-abdominal surgery, if enterocele is identified, McCall culdoplasty or the Moschcowitz or Halban procedure can be applied [60]. In 1912, Moschcowitz described a procedure involving the obliteration of the pouch of Douglas with several horizontal circular, purse-string-type sutures, beginning at the most distal part of the enterocele. The Halban procedure is the occlusion of the pouch of Douglas with several sagittal sutures positioned along the pouch in a vertical direction and was described in 1912 by Halban. McCall culdoplasty is the plication of the uterosacral ligament in the midline. Two or three sutures are placed. McCall reported no recurrent enterocele during a 3-year follow-up [66]. There are many modifications of the procedures that are a combination of the McCall, Moschcowitz, and Halban procedures.
Minimally invasive adaptations of this procedure have been developed, initially as laparoscopic, and more recently as robotic surgery. Laparoscopic technique is basically the same as an open procedure and the indication is also the same. However, the advantages of minimally invasive surgery, which include improved cosmetic aspects, reduced pain, short recovery time, low morbidity, and convenience of surgery, have led surgeons to perform more laparoscopic-based surgery in the field of POP. Most reports that compared open, laparoscopic, and robotic POP surgeries with one another showed similar outcomes in cure rate, anatomic results, and complications [16,67,68,69,70,71,72,73,74,75,76,77,78]. From the aspect of satisfaction with the operation, minimally invasive surgery showed a higher rate of satisfaction [78].
Generally, four port placements are used, including an umbilical camera port, and an average of 6 and 4 sutures are used for paravaginal repair and Burch operation, respectively [16]. Most of the reported data showed similar cure rates (62% to 100%) between laparoscopic Burch operation and open surgery [73,74,75,79]. Robotic surgery is also an emerging technique for cystocele repair [76,77]. Daneshgari et al. [76] reported the robotic abdominal sacrocolpopexy or sacrouteropexy repair of POP on the basis of POP-Q stage. Among the 12 patients, 9 patients (75%) successfully underwent robotic surgery. Postoperatively, mean POP-Q stage decreased to 0 from preoperative stage 3.1. In this study, early (6 months postoperatively) outcomes of the robotic approach showed results comparable with those of open or laparoscopic surgery. Rivoire et al. [78] reported 138 cases of laparoscopic promontofixation and anterior mesh repair for cystocele, apical prolapse, and rectocele. The meshes are placed in the vesicovaginal space, to the levator ani muscles, and in the prerectal space. Paravaginal repair or Burch operation is done to treat the anterior compartment in Retzius space. During the follow-up periods (mean, 33.7 months), 16 cases (12%) showed prolapse recurrence, and among them, repeat surgery was needed in 7 cases (5%). After the secondary repair, none of the patients had a recurrence more than 40 months after the procedure, and the probability of successful treatment remained as stable as 80%. For apical segment prolapse, the laparoscopic technique is well established. Wang et al. [80] reported the laparoscopic sacrospinous ligament fixation (LSSLF) technique and showed successful outcomes in safety as well as effectiveness (cure rate, 93.5%) for uterovaginal prolapse. This outcome is comparable to that of the open procedure (90.1% to 97%) [45,81,82].
The LSSLF technique is as below. After pneumoperitoneum is established, one umbilical 11-mm camera port, two 5-mm left lateral bythus ports, and one 5-mm right lateral bythus ports are placed. Then a laparoscopic hysterectomy is performed and entrance into the Retzius space is made 2 to 3 cm above the vesical reflection. Blunt dissection is performed until the ischial spine is identified to expose the sacrospinous ligament. With the use of 2-0 nonabsorbable polyester sutures, the needle is passed through the sacrospinous ligament and is then passed through the tip of the vaginal vault or cervix where the uterosacral ligament accretes. The vaginal vault tip should be fixed at a POP-Q level of -5 of -6.
Sergent et al. [69] reported the outcome of laparoscopic sacrocolpopexy with the use of polyester mesh for 124 genitourinary prolapse cases that presented with symptomatic upper vaginal prolapse or stage 2 apex prolapse with anterior or posterior vaginal wall prolapse. They used two separate meshes. The large Y shape mesh was attached to the bilateral levator ani muscle and then to the posterior vaginal wall. The small arrow-shaped anterior mesh was attached to the posterior portion of the bladder and then to the vagina and the uterine cervix. The surgical failure rate was 4.0% (n=5), the early postoperative (<6 weeks) complication rate was 28.5%, and the late complication rate was 8.4%. They reported anatomic cure rates of 97.4%, 88.8%, and 98.3% for apical, anterior, and posterior prolapse, respectively. Robotic sacrocolpopexy has been actively performed for prolapse in recent years and has been reported to be feasible and convenient with results comparable to those of laparoscopic surgery and even open surgery [70,71,83]. For robotic surgery, generally, a W-shaped configuration of 5 or more port placements is need. A camera port is placed at the level of the umbilicus and the full Trendelenburg position is required for pelvic visualization. The procedures are the same with open and laparoscopic surgery. Identification of the sacral promontory, identification of the anterior longitudinal ligament, and mesh fixation of nonabsorbable sutures is done sequentially. Robotic surgery is convenient when supracervical hysterectomy is done with prolapse surgery [83]. However, the learning curve associated with laparoscope-based surgery and the relatively high cost are reported as limitations of robotic surgery [70].
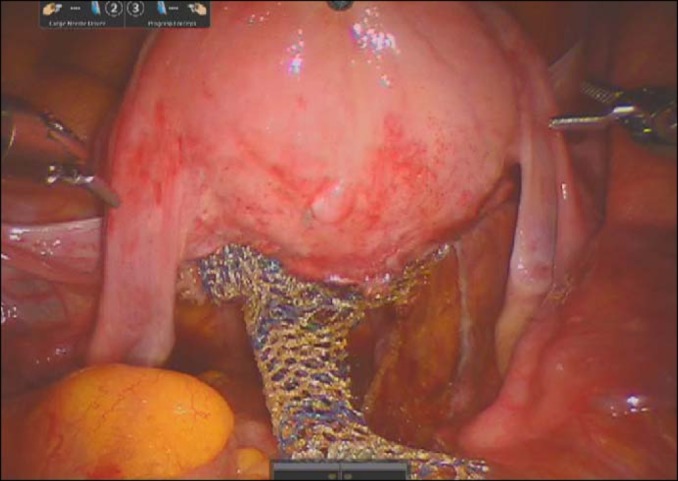
Because abdominal colpopexy shows a better outcome than vaginal colpopexy, robot-assisted abdominal sacrocolpopexy will be a next-generation standard treatment modality for total POP (Fig. 1). Robot-assisted abdominal sacrocolpopexy has the advantages of both abdominal surgery and minimally invasive surgery, including less invasiveness and low postoperative morbidity.
Restorative surgical repairs of POP are associated with high failure rates. In particular, anterior vaginal wall prolapse may recur in 30% to 70% of patients after standard anterior colporrhaphy [18,58,84], whereas recurrence rates in the posterior compartment after posterior colporrhaphy are only 10% to 20% [85,86]. In light of these high failure rates of the anterior compartment, vaginal surgery with synthetic meshes and biological grafts has been introduced.
Tension-free vaginal kits have been introduced to provide graft-augmented durable repair. The proposed advantages of the kits are a less invasive procedure, the standardization of technique, the standardization of the mesh, and the ability to repair multiple compartments through a vaginal approach. Most of the literature supports the use of transvaginal mesh in the anterior compartment but not in posterior or apical compartments [84]. The Cochrane review notes that in the anterior compartment, biological graft materials or synthetic mesh are better than no graft [62], and synthetic mesh is better than a biological graft in terms of the objective failure of the anterior compartment [87].
The indication of transvaginal mesh surgery for anterior compartment repair is the selection of patients who are at risk for native tissue failure and in whom the benefit of mesh use outweigh the risks. These groups include patients with recurrent prolapse, high-stage prolapse (POP-Q stage III or IV), collagen disorders, and chronic stress to the pelvic floor.
The procedures of commercially available vaginal kits are similar in many aspects. After midline vertical anterior vaginal wall incision, a full thickness vaginal flap is made. Sharp and blunt dissection towards both the ATFP and ischial spine is performed without disruption of the ATFP. Two helical trocars are passed through the median portion of the obturator foramen on both sides into the vaginal fields. The proximal and distal ends of the mesh are trimmed to fit the dissected space without redundancy and are secured to the endopelvic fascia and vaginal apex with delayed absorbable sutures. The body of the mesh is loosely tensioned [84].
Nguyen and Burchette [84] reported an 87% success rate in the mesh group, compared with 55% in the anterior colporrhaphy group (p=0.005), after 1 year of follow-up. The rates of de novo dyspareunia were 16% and 9% in the colporrhaphy and mesh groups, respectively. Vaginal mesh extrusion was noted in 5% of the patients. Another randomized controlled study demonstrated that recurrent stage II or III anterior vaginal prolapse occurred less often when anterior colporrhaphy was performed with mesh reinforcement (6.7% compared with 38.5%, p<0.001) [88].
The common complications of mesh-augmented repair are exposure of the mesh, dyspareunia, and vaginal contracture. The U.S. Food and Drug Administration (FDA) conducted a search of the Manufacturer and User Device Experience database for Medical Device Reports (MDRs) of adverse events associated with all urogynecological surgical mesh products received from January 1, 2005, to December 31, 2010. The search identified 3,979 reports of injury, death, and malfunction. Among the 3,979 reports, 2,874 reports were received in the last 3 years (January 2008 through December 31, 2010) and included 1,503 reports associated with POP repairs and 1,371 associated with stress urinary incontinence repairs. The number of MDRs associated with POP repairs increased by more than fivefold compared with the number of reports received in the previous 3 years (January 1, 2005, through December 31, 2007). From 2008 to 2010, the most frequent complications reported to the FDA from the use of surgical mesh devices for POP repair included vaginal mesh exposure, pain including dyspareunia, infection, urinary problems, bleeding, and organ perforation. There were also reports of recurrent prolapse, neuromuscular problems, vaginal scarring or shrinkage, and emotional problems. There were seven reported deaths associated with POP repairs. Three of the deaths associated with prolapse repair were related to the mesh placement procedure (two bowel perforations, one hemorrhage). Four deaths were due to postoperative complications not directly related to the mesh placement procedure [89].
Recently, the FDA issued a safety communication pertaining to the mesh used for prolapse and not a sling. The American Urological Association issued a position statement noting that extensive data support the use of mesh slings for stress urinary incontinence [90]. With respect to transvaginal mesh for prolapse repair, the Association noted that many have successfully used mesh, although there are inherent complications associated with its use and that a thorough informed consent discussion between the surgeon and the patient should occur [91].
Colpocleisis has advantages in elderly patients with multiple comorbidities and who do not desire sexual activity. This procedure has other advantages, such as a short operation time and the ease and safety of employing regional or local anesthesia, minimizing the uncommon complications of hemorrhage or nerve injury.
These procedures include partial or total colpocleisis and colpectomy. In the absence of a uterus, either complete colpocleisis or colpectomy can be performed, whereas the presence of the uterus necessitates partial colpocleisis, which creates two lateral channels to allow uterine drainage. The most commonly described technique for partial colpocleisis is a variation of the operation originally described by LeFort in 1977 [92,93].
Other simplified procedures include a rectangular incision of the anterior vaginal wall from 2 cm below the external urethral meatus to the bladder neck until the pubocervical fascia is identified. In the same manner, the posterior vaginal wall, which is exactly opposite the site of anterior incision, and tissue are dissected until the prerectal fascia is exposed. Interrupted 2-0 absorbable sutures are made on the dependent vaginal wall. This procedure can be performed under local anesthesia and on an outpatient basis [94].
The treatment modality should be chosen according to the patient's medical status, anatomic defects, balance between the benefits and risks, patient's preference, and the surgeon's experience. If the surgeon and patient choose compensatory surgery with synthetic meshes, informed consent should be obtained in consideration of mesh-related complications. The cure rate will be acceptable if the above-mentioned points are considered. The prevalence of POP will increase with the growing aged population. More extensive studies are needed for prevention and proper treatment.
ACKNOWLEDGMENTS
We thank Jin Ho Hwang and Young Dong Yu (Department of Urology, CHA Bundang Medical Center) for their contribution to correcting data and English editing.
References
1. Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2001; 98:646–651. PMID: 11576582.

2. Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997; 89:501–506. PMID: 9083302.

3. Hagen S, Stark D, Maher C, Adams E. Conservative management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2004; (2):CD003882. PMID: 15106225.

4. Kegel AH. Progressive resistance exercise in the functional restoration of the perineal muscles. Am J Obstet Gynecol. 1948; 56:238–248. PMID: 18877152.

5. Kisner C, Colby LA. Therapeutic exercise: foundations and tchniques. 4th ed. Philadelphia: F.A. Davis Co;2003.
6. Deger RB, Menzin AW, Mikuta JJ. The vaginal pessary: past and present. Postgrad Obstet Gynecol. 1993; 13:1–8.
7. Clemons JL, Aguilar VC, Tillinghast TA, Jackson ND, Myers DL. Risk factors associated with an unsuccessful pessary fitting trial in women with pelvic organ prolapse. Am J Obstet Gynecol. 2004; 190:345–350. PMID: 14981372.

8. Sulak PJ, Kuehl TJ, Shull BL. Vaginal pessaries and their use in pelvic relaxation. J Reprod Med. 1993; 38:919–923. PMID: 8120847.

9. Wu V, Farrell SA, Baskett TF, Flowerdew G. A simplified protocol for pessary management. Obstet Gynecol. 1997; 90:990–994. PMID: 9397117.

10. Clemons JL, Aguilar VC, Tillinghast TA, Jackson ND, Myers DL. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004; 190:1025–1029. PMID: 15118635.

11. Citgez S, Demirkesen O, Ozdemir F, Gevher F, Demirdag C, Onal B, et al. Transvaginal repair using acellular collagen biomesh for the treatment of anterior prolapse. Urol J. 2014; 11:1271–1277. PMID: 24595936.
12. Delroy CA, Castro Rde A, Dias MM, Feldner PC Jr, Bortolini MA, Girao MJ, et al. The use of transvaginal synthetic mesh for anterior vaginal wall prolapse repair: a randomized controlled trial. Int Urogynecol J. 2013; 24:1899–1907. PMID: 23632800.

13. Young SB, Daman JJ, Bony LG. Vaginal paravaginal repair: one-year outcomes. Am J Obstet Gynecol. 2001; 185:1360–1366. PMID: 11744910.

14. Bruce RG, El-Galley RE, Galloway NT. Paravaginal defect repair in the treatment of female stress urinary incontinence and cystocele. Urology. 1999; 54:647–651. PMID: 10510922.

15. Scotti RJ, Garely AD, Greston WM, Flora RF, Olson TR. Paravaginal repair of lateral vaginal wall defects by fixation to the ischial periosteum and obturator membrane. Am J Obstet Gynecol. 1998; 179(6 Pt 1):1436–1445. PMID: 9855578.

16. Miklos JR, Kohli N. Laparoscopic paravaginal repair plus burch colposuspension: review and descriptive technique. Urology. 2000; 56(6 Suppl 1):64–69. PMID: 11114565.

17. Porges RF, Smilen SW. Long-term analysis of the surgical management of pelvic support defects. Am J Obstet Gynecol. 1994; 171:1518–1526. PMID: 7802061.

18. Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001; 185:1299–1304. PMID: 11744900.

19. Chesson RR, Schlossberg SM, Elkins TE, Menefee S, McCammon K, Franco N, et al. The use of fascia lata graft for correction of severe or recurrent anterior vaginal wall defects. J Pelvic Surg. 1999; 5:96–103.
20. Sand PK, Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Am J Obstet Gynecol. 2001; 184:1357–1362. PMID: 11408853.

21. Natale F, La Penna C, Padoa A, Agostini M, De Simone E, Cervigni M. A prospective, randomized, controlled study comparing Gynemesh, a synthetic mesh, and Pelvicol, a biologic graft, in the surgical treatment of recurrent cystocele. Int Urogynecol J Pelvic Floor Dysfunct. 2009; 20:75–81. PMID: 18923805.

22. Altman D, Vayrynen T, Engh ME, Axelsen S, Falconer C. Nordic Transvaginal Mesh Group. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med. 2011; 364:1826–1836. PMID: 21561348.

23. Lovatsis D, Drutz HP. Is transabdominal repair of mild to moderate cystocele necessary for correction of prolapse during a modified Burch procedure? Int Urogynecol J Pelvic Floor Dysfunct. 2001; 12:193–198. PMID: 11451008.

24. Withagen MI, Milani AL, den Boon J, Vervest HA, Vierhout ME. Trocar-guided mesh compared with conventional vaginal repair in recurrent prolapse: a randomized controlled trial. Obstet Gynecol. 2011; 117(2 Pt 1):242–250. PMID: 21252735.

25. Rodriguez LV, Bukkapatnam R, Shah SM, Raz S. Transvaginal paravaginal repair of high-grade cystocele central and lateral defects with concomitant suburethral sling: report of early results, outcomes, and patient satisfaction with a new technique. Urology. 2005; 66(5 Suppl):57–65. PMID: 16194709.

26. Walters MD. Pelvic floor prolapse: cystocele and rectocele. In : Walters MD, Karram MM, editors. Clinical urogynecology. St. Louis: Mosby-Year Book;1993. p. 225–236.
27. Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol. 1993; 36:976–983. PMID: 8293598.

28. Francis WJ, Jeffcoate TN. Dyspareunia following vaginal operations. J Obstet Gynaecol Br Commonw. 1961; 68:1–10. PMID: 13701244.

29. Kahn MA, Stanton SL. Posterior colporrhaphy: its effects on bowel and sexual function. Br J Obstet Gynaecol. 1997; 104:82–86. PMID: 8988702.
30. Lim YN, Muller R, Corstiaans A, Hitchins S, Barry C, Rane A. A long-term review of posterior colporrhaphy with Vypro 2 mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2007; 18:1053–1057. PMID: 17216133.

31. de Tayrac R, Devoldere G, Renaudie J, Villard P, Guilbaud O, Eglin G, et al. Prolapse repair by vaginal route using a new protected low-weight polypropylene mesh: 1-year functional and anatomical outcome in a prospective multicentre study. Int Urogynecol J Pelvic Floor Dysfunct. 2007; 18:251–256. PMID: 16699914.

32. Schmidlin-Enderli K, Schuessler B. A new rectovaginal fascial plication technique for treatment of rectocele with obstructed defecation: a proof of concept study. Int Urogynecol J. 2013; 24:613–619. PMID: 22890282.

33. Maher CF, Qatawneh AM, Baessler K, Schluter PJ. Midline rectovaginal fascial plication for repair of rectocele and obstructed defecation. Obstet Gynecol. 2004; 104:685–689. PMID: 15458886.

34. Singh K, Cortes E, Reid WM. Evaluation of the fascial technique for surgical repair of isolated posterior vaginal wall prolapse. Obstet Gynecol. 2003; 101:320–324. PMID: 12576256.

35. Nichols DH, Randall CL. Posterior colporrhaphy and perineorrhaphy. In : Nichols DH, Randal CL, editors. Vaginal surgery. 4th ed. Baltimore: Lippincortt Williams & Wilkins;1996. p. 257–289.
36. Abramov Y, Gandhi S, Goldberg RP, Botros SM, Kwon C, Sand PK. Site-specific rectocele repair compared with standard posterior colporrhaphy. Obstet Gynecol. 2005; 105:314–318. PMID: 15684158.

37. Glavind K, Madsen H. A prospective study of the discrete fascial defect rectocele repair. Acta Obstet Gynecol Scand. 2000; 79:145–147. PMID: 10696964.

38. Cundiff GW, Weidner AC, Visco AG, Addison WA, Bump RC. An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol. 1998; 179(6 Pt 1):1451–1456. PMID: 9855580.

39. Kenton K, Shott S, Brubaker L. Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol. 1999; 181:1360–1363. PMID: 10601913.

40. Porter WE, Steele A, Walsh P, Kohli N, Karram MM. The anatomic and functional outcomes of defect-specific rectocele repairs. Am J Obstet Gynecol. 1999; 181:1353–1358. PMID: 10601912.

41. Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006; 195:1762–1771. PMID: 17132479.

42. Randall CL, Nichols DH. Surgical treatment of vaginal inversion. Obstet Gynecol. 1971; 38:327–332. PMID: 5094313.
43. Meuman N, Natalia S, Vladimir S, Jacob B. Anterior needle-guided mesh in advanced pelvic organ prolapse: apical fixation on sacrospinous ligaments. Eur J Obstet Gynecol Reprod Biol. 2014; 172:120–123. PMID: 24210791.
44. Hefni MA, El-Toukhy TA. Long-term outcome of vaginal sacrospinous colpopexy for marked uterovaginal and vault prolapse. Eur J Obstet Gynecol Reprod Biol. 2006; 127:257–263. PMID: 16377061.

45. Nichols DH. Massive eversion of the vagina. In : Nichols DH, editor. Gynecologic and obstetric surgery. St. Louis: Mosby;1993. p. 431–464.
46. Nieminen K, Huhtala H, Heinonen PK. Anatomic and functional assessment and risk factors of recurrent prolapse after vaginal sacrospinous fixation. Acta Obstet Gynecol Scand. 2003; 82:471–478. PMID: 12752079.

47. Karram MM, Sze EH, Walters MD. Surgical treatment of vaginal vault prolapse. In : Walters MD, Karram MM, editors. Urogynecology and reconstructive pelvic surgery. 2nd ed. St. Louis: Mosby;1999. p. 235–256.
48. Paraiso MF, Ballard LA, Walters MD, Lee JC, Mitchinson AR. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1996; 175:1423–1430. PMID: 8987920.

49. Thompson JR, Gibb JS, Genadry R, Burrows L, Lambrou N, Buller JL. Anatomy of pelvic arteries adjacent to the sacrospinous ligament: importance of the coccygeal branch of the inferior gluteal artery. Obstet Gynecol. 1999; 94:973–977. PMID: 10576185.

50. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000; 183:1365–1373. PMID: 11120498.

51. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000; 183:1402–1410. PMID: 11120503.

52. Cosma S, Menato G, Preti M, Petruzzelli P, Tin MC, Riboni F, et al. Advanced utero-vaginal prolapse and vaginal vault suspension: synthetic mesh vs native tissue repair. Arch Gynecol Obstet. 2014; 289:1053–1060. PMID: 24305747.

53. Meeks GR, Washburne JF, McGehee RP, Wiser WL. Repair of vaginal vault prolapse by suspension of the vagina to iliococcygeus (prespinous) fascia. Am J Obstet Gynecol. 1994; 171:1444–1452. PMID: 7802052.
54. Maher CF, Murray CJ, Carey MP, Dwyer PL, Ugoni AM. Iliococcygeus or sacrospinous fixation for vaginal vault prolapse. Obstet Gynecol. 2001; 98:40–44. PMID: 11430954.

55. Medina CA, Croce C, Candiotti K, Takacs P. Comparison of vaginal length after iliococcygeus fixation and sacrospinous ligament fixation. Int J Gynaecol Obstet. 2008; 100:267–270. PMID: 18022624.

56. Petros PE. Vault prolapse II: restoration of dynamic vaginal supports by infracoccygeal sacropexy, an axial day-case vaginal procedure. Int Urogynecol J Pelvic Floor Dysfunct. 2001; 12:296–303. PMID: 11715994.
57. Farnsworth BN. Posterior intravaginal slingplasty (infracoccygeal sacropexy) for severe posthysterectomy vaginal vault prolapse: a preliminary report on efficacy and safety. Int Urogynecol J Pelvic Floor Dysfunct. 2002; 13:4–8. PMID: 11999204.
58. Sivaslioglu AA, Ilhan TT, Aydogmus S, Uzun M, Dolen I. The comparison of the anatomical and symptomatic outcomes of sacrocolpopexy and posterior intravaginal slingoplasty. Int Urogynecol J. 2011; 22:1363–1368. PMID: 21562912.

59. Lane FE. Repair of posthysterectomy vaginal-vault prolapse. Obstet Gynecol. 1962; 20:72–77. PMID: 14462011.

60. Shull BL, Bachofen CG. Enterocele and rectocele. In : Walters MD, Karram MM, editors. Urogynecology and reconstructive pelvic surgery. 2nd ed. St. Louis: Mosby;1999. p. 221–234.
61. Nygaard IE, McCreery R, Brubaker L, Connolly A, Cundiff G, Weber AM, et al. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004; 104:805–823. PMID: 15458906.

62. Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women: a short version Cochrane review. Neurourol Urodyn. 2008; 27:3–12. PMID: 18092333.

63. Tulikangas PK, Piedmonte MR, Weber AM. Functional and anatomic follow-up of enterocele repairs. Obstet Gynecol. 2001; 98:265–268. PMID: 11506843.

64. Waters EG. Vaginal prolapse; technic for correction and prevention at hysterectomy. Obstet Gynecol. 1956; 8:432–436. PMID: 13370010.
65. Wheeless CR Jr. Vaginal repair of enterocele. In : Wheeless CR, editor. Atlas of pelvic surgery. 3rd ed. Baltimore: Williams & Wilkins;1997. p. 56–61.
66. McCall ML. Posterior culdeplasty; surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957; 10:595–602. PMID: 13484166.
67. Crigler B, Zakaria M, Hart S. Total laparoscopic hysterectomy with laparoscopic uterosacral ligament suspension for the treatment of apical pelvic organ prolapse. Surg Technol Int. 2012; 22:195–202. PMID: 23225594.
68. Koyama M, Yoshida S, Koyama S, Ogita K, Kimura T, Shimoya K, et al. Surgical reinforcement of support for the vagina in pelvic organ prolapse: concurrent iliococcygeus fascia colpopexy (Inmon technique). Int Urogynecol J Pelvic Floor Dysfunct. 2005; 16:197–202. PMID: 15875235.

69. Sergent F, Resch B, Loisel C, Bisson V, Schaal JP, Marpeau L. Mid-term outcome of laparoscopic sacrocolpopexy with anterior and posterior polyester mesh for treatment of genito-urinary prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 156:217–222. PMID: 21353736.

70. Elliott DS, Frank I, Dimarco DS, Chow GK. Gynecologic use of robotically assisted laparoscopy: Sacrocolpopexy for the treatment of high-grade vaginal vault prolapse. Am J Surg. 2004; 188(4A Suppl):52S–56S. PMID: 15476652.

71. Kramer BA, Whelan CM, Powell TM, Schwartz BF. Robot-assisted laparoscopic sacrocolpopexy as management for pelvic organ prolapse. J Endourol. 2009; 23:655–658. PMID: 19335154.

72. Wong MT, Meurette G, Rigaud J, Regenet N, Lehur PA. Rbotic versus laparoscopic rectopexy for complex rectocele: a prospective comparison of short-term outcomes. Dis Colon Rectum. 2011; 54:342–346. PMID: 21304307.
73. el-Toukhy TA, Davies AE. The efficacy of laparoscopic mesh colposuspension: results of a prospective controlled study. BJU Int. 2001; 88:361–366. PMID: 11564022.

74. Valpas A, Kivela A, Penttinen J, Kauko M, Kujansuu E, Tomas E, et al. Tension-free vaginal tape and laparoscopic mesh colposuspension in the treatment of stress urinary incontinence: immediate outcome and complications: a randomized clinical trial. Acta Obstet Gynecol Scand. 2003; 82:665–671. PMID: 12790850.
75. Prezioso D, Iacono F, Di Lauro G, Illiano E, Romeo G, Ruffo A, et al. Stress urinary incontinence: long-term results of laparoscopic Burch colposuspension. BMC Surg. 2013; 13(Suppl 2):S38. PMID: 24268031.

76. Daneshgari F, Kefer JC, Moore C, Kaouk J. Robotic abdominal sacrocolpopexy/sacrouteropexy repair of advanced female pelvic organ prolaspe (POP): utilizing POP-quantification-based staging and outcomes. BJU Int. 2007; 100:875–879. PMID: 17822467.

77. Francis SL, Agrawal A, Azadi A, Ostergard DR, Deveneau NE. Robotic Burch colposuspension: a surgical case and instructional video. Int Urogynecol J. 2014; 7. 17. [Epub]. http://dx.doi.org/10.1007/s00192-014-2471-1.

78. Rivoire C, Botchorishvili R, Canis M, Jardon K, Rabischong B, Wattiez A, et al. Complete laparoscopic treatment of genital prolapse with meshes including vaginal promontofixation and anterior repair: a series of 138 patients. J Minim Invasive Gynecol. 2007; 14:712–718. PMID: 17980331.

79. Gunn GC, Cooper RP, Gordon NS, Gagnon L. Use of a new device for endoscopic suturing in the laparoscopic Burch procedure. J Am Assoc Gynecol Laparosc. 1994; 2:65–70. PMID: 9050535.

80. Wang Y, Wang D, Li Y, Liang Z, Xu H. Laparoscopic sacrospinous ligament fixation for uterovaginal prolapse: experience with 93 cases. Int Urogynecol J. 2011; 22:83–89. PMID: 20740359.

81. Morley GW, DeLancey JO. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988; 158:872–881. PMID: 3364499.

82. Sze EH, Karram MM. Transvaginal repair of vault prolapse: a review. Obstet Gynecol. 1997; 89:466–475. PMID: 9052607.

83. Rosenblum N. Robotic approaches to prolapse surgery. Curr Opin Urol. 2012; 22:292–296. PMID: 22647648.

84. Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008; 111:891–898. PMID: 18378748.
85. Kahn MA, Stanton SL, Kumar D, Fox SD. Posterior colporrhaphy is superior to the transanal repair for the treatment of posterior vaginal wall prolapse [abstract]. Neurourol Urodyn. 1999; 18:329–330.
86. Milani AL, Withagen MI, Schweitzer KJ, Janszen EW, Vierhout ME. Midline fascial plication under continuous digital transrectal control: which factors determine anatomic outcome? Int Urogynecol J. 2010; 21:623–630. PMID: 20146055.

87. Yurteri-Kaplan LA, Gutman RE. The use of biological materials in urogynecologic reconstruction: a systematic review. Plast Reconstr Surg. 2012; 130(5 Suppl 2):242S–253S. PMID: 23096979.
88. Hiltunen R, Nieminen K, Takala T, Heiskanen E, Merikari M, Niemi K, et al. Low-weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol. 2007; 110(2 Pt 2):455–462. PMID: 17666627.
89. FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary [Internet]. Silver Spring, MD: U.S. Food and Drug Administration;cited 2014 Nov 5. Available from: http://www.fda.gov/medicaldevices/safety/alertsandnotices/publichealthnotifications/ucm061976.htm.
90. AUA position statement on the use of vaginal mesh for the surgical treatment of stress urinary incontinence [Internet]. Linthicum, MD: American Urological Association;cited 2014 Nov 5. Available from: http://www.auanet.org/about/vaginal-mesh-for-sui.cfm.
91. AUA position statement on the use of vaginal mesh for the repair of pelvic organ prolapse [Internet]. Linthicum, MD: American Urological Association;cited 2014 Nov 5. Available from: http://www.auanet.org/about/vaginal-mesh-for-pelvic-organ-prolapse.cfm.
92. Goldman J, Ovadia J, Feldberg D. The Neugebauer-Le Fort operation: a review of 118 partial colpocleises. Eur J Obstet Gynecol Reprod Biol. 1981; 12:31–35. PMID: 7195840.

93. Langmade CF, Oliver JA Jr. Partial colpocleisis. Am J Obstet Gynecol. 1986; 154:1200–1205. PMID: 3717230.

94. Kovac SR, Zimmerman CW, editors. Advances in reconstructive vaginal surgery. Philadelphia: Lippincott Williams & Wilkins;2007.
95. DeLancey JO, Morley GW. Total colpocleisis for vaginal eversion. Am J Obstet Gynecol. 1997; 176:1228–1232. PMID: 9215178.

96. Ridley JH. Evaluation of the colpocleisis operation: a report of fifty-eight cases. Am J Obstet Gynecol. 1972; 113:1114–1119. PMID: 4635182.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download