Abstract
Purpose
To analyze the effectiveness of tamsulosin 0.2 mg once daily for 3 months according to the degree of intravesical prostatic protrusion (IPP) in patients with benign prostatic hyperplasia (BPH).
Materials and Methods
A total of 134 BPH patients over 40 years of age treated with tamsulosin 0.2 mg between January 2007 and January 2009 were enrolled retrospectively. The patients were classified into three groups according to the degree of IPP: below 5 mm (group A), between 5 and 10 mm (group B), and over 10 mm (group C). Prostate volume, prostate-specific antigen (PSA), prostatic urethral length (PUL), and prostatic adenoma urethral length (PAUL) were evaluated before treatment. International Prostate Symptom Score and Quality of Life (IPSS/QoL), maximal urine flow rate (Qmax), and postvoid residual (PVR) volume were measured before treatment, and improvement in the three groups was compared after 3 months.
Results
The mean age of the patients was 65.01±7.38 years. Mean IPPs were 0.90±1.39 mm (group A, n=90), 6.92±1.10 mm (group B, n=24), and 16.60±4.06 mm (group C, n=20). Prostate volume, PUL, PAUL, PSA, Qmax, and PVR showed significant correlations with IPP (p<0.05), but not with IPSS/QoL score (p>0.05). Comparison of parameters before and after 3 months showed that medication improved total IPSS and subscores (p<0.001), QoL (p<0.001), Qmax (p<0.001), and PVR (p=0.030) in group A. In group B, it improved total IPSS (p=0.01), irritative subscore (p<0.001), and obstructive subscore (p=0.03). In group C, only total IPSS (p=0.01) and irritative score (p<0.001) were significantly improved.
Benign prostatic hyperplasia (BPH) is a progressive disease that has been on the rise in men over 50 [1]. The incidence of BPH is thought to rapidly increase in an aging society.
Transrectal ultrasonography (TRUS) is widely used because it can estimate prostate volume, shape, and the presence of adenoma and can evaluate anatomical structure through the application of noninvasive methods. Furthermore, TRUS permits a more accurate evaluation of the prostate than does computed tomography or magnetic resonance imaging. Intravesical prostatic protrusion (IPP) is the result of morphological changes leading to protrusion of hypertrophied prostate tissue into the bladder. It is known that more extensive IPP can lead to increased voiding symptoms by causing more serious bladder outlet obstruction (BOO) [2-4]. The most accurate method for diagnosing BOO is a pressure flow study (PFS). However, noninvasive methods are being sought as substitutes, because of the invasiveness, cost, and morbidity associated with PFS. Measurement of IPP has the advantages that it is reproducible, has parameters and correlations established by conventional PFS, and does not require urination during the test [5].
It is reported that increased IPP due to an enlarged prostate may aggravate storage symptoms as a consequence of elongation of the prostatic urethra and increased stimulation of the bladder neck and trigone. Moreover, the increased IPP can affect storage symptoms more than voiding symptoms owing to stimulation of the bladder [6-9]. Therefore, the aim of this work was to examine the effect of different IPP levels as estimated by TRUS on changes in the voiding and storage symptoms of BPH patients, the general progress of patients after daily administration of tamsulosin 0.2 mg, and the effectiveness of the medication.
The study included 134 men over 40 years of age with lower urinary tract symptoms (LUTS) who visited our clinic between January 2007 and January 2009, retrospectively. All men underwent urinalysis, routine laboratory tests, measurement of prostate-specific antigen (PSA), uroflowmetry (Urodyn-1000; Medtronic Inc., West Palm Beach, FL, USA), 6.5 MHz probe TRUS (SA-8000, Medison, Seoul, Korea), and measurement of post-voided residual (PVR) volume by ultrasonography. In patients with PSA≥4 ng/ml, we performed TRUS-guided biopsies to rule out prostate cancer and enrolled the patients who were not diagnosed with prostate cancer. Degrees of initial International Prostate Symptom Scores and Quality of Life (IPSS/QoL) score, prostate volume, maximal urine flow rate (Qmax), and PVR were not considered as exclusion criteria. The following subjects were excluded from the study: those who had histories of gross hematuria, urinary tract infection, urinary tract stone disease, or pelvic surgery; those with a diagnosis of neurogenic bladder or urethral stricture; and those using anticholinergic agents and 5-alpha-reductase inhibitors. Using TRUS, retrospectively, we identified the bladder neck and protrusion of the prostate into the bladder according to the classification system of IPP as used by Nose et al. [5]. By measuring the vertical distance from the tip of the protrusion to the circumference of the bladder at the base of the prostate gland, we divided the patients into 3 groups according to the extent of IPP: those with an IPP of 5 mm or less (group A), those with an IPP of 5 to 10 mm (group B), and those with an IPP of greater than 10 mm (group C) (Fig. 1). Prostate volume, transitional zone volume (TZV), prostatic urethral length (PUL), and prostatic adenoma urethral length (PAUL) were estimated by TRUS. PUL and PAUL were measured as the vertical distance from the base of the prostate gland to the apex of the prostate gland and to the apex of the prostate adenoma, respectively, by retrospective review. IPSS/QoL scores were obtained for all patients, and IPSS scores were subdivided according to irritative subscore and obstructive subscore. The scores on the first visit were compared with those obtained after administration of tamsulosin 0.2 mg for 3 months. Uroflowmetry measurements were also compared before and after tamsulosin administration.
Each characteristic was compared among the groups by one-way analysis of variance test. The relation of each variable with IPP was examined by correlation and multiple linear regression analysis. The IPSS/QoL, Qmax, and PVR before and after tamsulosin 0.2 mg administration were compared by using Student's paired t-test. Statistical analyses were performed with Open Office.org Calc ver. 3.2.1 (Oracle Co., Redwood Shores, CA, USA) and MedCalc ver. 11.2.1.0 (MedCalc Software, Mariakerke, Belgium). A p<0.05 was considered statistically significant.
The mean age of the patients was 65.01±7.38 years. Mean prostate volume and mean TZV were 47.11±21.45 ml and 21.57±14.75 ml, respectively. Mean PSA was 4.26±4.34 ng/ml. Mean IPSS total score, irritative subscore, obstructive subscore, and QoL were 18.72±7.17, 7.46±3.37, 11.27±4.47, and 3.87±1.08, respectively.
Mean Qmax was 10.86±4.99 ml/s and mean PVR was 77.36±78.71 ml. Mean IPP was 4.31±5.96 mm, mean PUL was 4.56±0.08 cm, and mean PAUL was 3.39±0.82 cm. Average IPP values of the groups were as follows: group A, 0.90±1.39 mm; group B, 6.92±1.10 mm; group C, 16.60±4.06 mm. Among the three groups, all characteristics showed statistical significance (Table 1) not including age and PSA density. Correlation analysis indicated that prostate volume (r=0.56, p<0.001), PUL (r=0.63, p<0.001), PAUL (r=0.61, p<0.001), and PSA (r=0.22, p=0.01) varied with IPP. The same was true for Qmax (r=-0.28, p=0.001) and PVR (r=0.37, p<0.001), whereas IPSS total scores and subscores and QoL were unrelated to IPP before tamsulosin 0.2 mg administration (Table 2).
Table 3 compares IPSS total scores, subscores, QoL, Qmax, and PVR in the groups before and after administration of 0.2 mg tamsulosin for 3 months. In the total patients, IPSS total score (p<0.001), subscores (p<0.001), QoL (p<0.001), and Qmax (p<0.001) were significantly improved after treatment. However, PVR did not show statistical significance. In group A, IPSS total score (p<0.001), subscores (p<0.001), QoL (p<0.001), Qmax (p<0.001), and PVR (p=0.03) were significantly reduced after treatment. In group B, the same was true for IPSS total score (p=0.01), irritative subscore (p<0.001), and obstructive subscore (p=0.03), but not for QoL (p=0.15), Qmax (p=0.25), or PVR (p=0.39). Whereas in group C, only the IPSS total score (p=0.01) and IPSS irritative subscore (p<0.001) were significantly lower. By multiple linear regression analysis to evaluate IPP as a predictive factor, only IPP was statistically significantly related to whether both IPSS and Qmax were improved (IPSS, p=0.044; Qmax, p<0.001) (Table 4).
IPP refers to a morphological change in which the prostate protrudes into the bladder during the process of prostatic enlargement [10]. A median lobe of prostate tissue can increase bladder outlet resistance by causing a 'valve ball' type of BOO with incomplete opening of the bladder neck and disruption of its funneling effect [11]. The IPSS questionnaire and measurements of PSA, prostate volume, urine flow, and PVR can be useful in deciding on the treatment for LUTS/BPH; however, it is difficult to decide between medication and surgical treatment solely on the basis of these tests. In addition, the existence of BOO needs to be demonstrated. PFS is the reference standard for diagnosing BOO and differentiating this condition from detrusor underactivity [12]. However, the need for PFS in patients with BPH has been questioned. PFS cannot be applied to all patients because of its invasiveness, the discomfort that can be caused, and the possibility of infection. Noninvasive methods, including IPSS/QoL, Qmax, PVR, prostate volume, and IPP have been studied as potential substitutes for PFS. The European Association of Urology guidelines on the assessment of BPH recommend that PFS should be used only in patients with a voided volume of less than 150 ml, patients with a maximum urine flow greater than 15 ml/s prior to surgery, patients who are very young (<50 years) or very old (>80 years), and patients with postvoid residual urinary volumes greater than 300 ml, suspicion of neurogenic bladder dysfunction, postradical pelvic surgery, or previous unsuccessful invasive treatment of BPH [13]. Recently, TRUS and IPP have been separately proposed as useful noninvasive parameters for predicting BOO in patients with LUTS/BPH [14].
Chia et al. [11] suggested that IPP may be a diagnostic tool for predicting BOO in patients with LUTS/BPH. They found that grade III IPP correctly identified 94% of obstructed patients. Nose et al. [5] showed that the IPP grading system and Doppler urodynamic study have high sensitivities and specificities in the prediction of BOO. Lim et al. [2] who analyzed 95 patients with LUTS/BPH and associated IPP, concluded that only IPP was independently associated with BOO. However, they reported that severe IPP of >10 mm correctly predicted 71% of patients with BOO, whereas IPP of ≤10 mm identified only 61% of patients without BOO. Keqin et al. [15] analyzed 206 patients with enlarged prostates. They concluded that IPP was positively correlated with prostate volume, detrusor overactivity, bladder compliance, detrusor pressure at Qmax, BOO index, and PVR. Recently, Kim and Kim [16] investigated the correlation between degree of IPP and IPSS/QoL in 179 patients. They concluded that overactive bladder may be correlated with IPP. In another study, no significant correlation was observed between IPP grading and initial IPSS score; however, the evolution of the IPSS score was helpful in assessing clinical progression [17]. In our study, baseline IPSS/QoL scores did not show significant correlation with IPP grades among all groups. However, in comparison with end-point IPSS, percentage improvement in the IPSS score was higher in group A than in groups B and C (39.91% vs. 29.05% vs. 16.70%). In the present work, we found that the number of factors improved by tamsulosin 0.2 mg decreased in patients with higher IPP grades. According to previous studies, IPP is significantly correlated with increased prostate volume, transitional zone volume, PSA, decreased Qmax, and increased PVR. IPP has also been shown to be correlated with worsening of urination symptoms by BOO. In our patients, medication with tamsulosin yielded better responses in patients with lower IPP grades, and there was greater resistance to treatment in patients with higher grades. Furthermore, objective measurements did not show any improvements in the patients with higher IPP grades. In relation to PUL and PAUL in LUTS/BPH patients, no studies have yet been reported. As IPP increases, PUL and PAUL might concomitantly increase and thus were evaluated in our study. In the correlation analysis, PUL and PAUL showed a positive correlation with degree of IPP (PUL, <0.001; PAUL, <0.001).
Despite these advantages, IPP does not replace established parameters used in the clinical evaluation of BPH, such as IPSS/QoL, uroflowmetry, PVR, and prostate volume. IPP provides additional clinical information for predicting obstruction without the need for routine PFS. Also, IPP is not correlated with initial IPSS scores. However, it might help in devising treatment plans because of its advantages over other measurements, not its limitations.
There have been many studies of the correlation between IPP and BOO. However, these generally focused on the relation between the morphology of the prostate and BOO. In our study, we focused on clinical progression and found that in the patients with higher IPP grades, clinical symptoms, especially obstructive symptoms, were resistant to the administration of tamsulosin 0.2 mg for 3 months.
We acknowledge the potential limitations of this study: it was a retrospective study, the study population was small, and the enrolled patients did not undergo PFS, which is the standard method for distinguishing between BOO and detrusor contractility. In addition, measurement of IPP, PUL, and PAUL was performed retrospectively and may have induced several biases in our study. However, despite the possible bias, the results of this study suggest that IPP may help in evaluating LUTS/BPH and in deciding on a therapeutic plan with medical or surgical treatment.
IPP shows significant correlations with increased prostate volume, PUL, PAUL, PSA, decreased Qmax, and increased PVR. Patients with different levels of IPP differed with respect to the benefits achieved by a tamsulosin dose of 0.2 mg for 3 months. IPP was correlated with obstructive symptoms, and improvements of obstructive symptoms, Qmax, and PVR were more resistant to medical treatment as the level of IPP increased.
Figures and Tables
FIG. 1
Grading system for intravesical prostatic protrusion (IPP). (A) Grade I (<5 mm), (B) Grade II (5-10 mm), and (C) Grade III (>10 mm) IPP. IPP, intravesical prostatic protrusion.
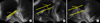
TABLE 2
Correlations between intravesical prostatic protrusion (IPP) and prostate volume/International Prostate Symptom Score (IPSS)/uroflowmetry parameters before tamsulosin 0.2 mg administration

TABLE 3
Comparison of IPSS total score and subscores, QoL, Qmax, and PVR in Groups A, B, and C after 3 months tamsulosin 0.2 mg administration

References
1. Chute CG, Panser LA, Girman CJ, Oesterling JE, Guess HA, Jacobsen SJ, et al. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol. 1993. 150:85–89.
2. Lim KB, Ho H, Foo KT, Wong MY, Fook-Chong S. Comparison of intravesical prostatic protrusion, prostate volume and serum prostatic-specific antigen in the evaluation of bladder outlet obstruction. Int J Urol. 2006. 13:1509–1513.
3. Reis LO, Barreiro GC, Baracat J, Prudente A, D'Ancona CA. Intravesical protrusion of the prostate as a predictive method of bladder outlet obstruction. Int Braz J Urol. 2008. 34:627–633.
4. Kim BH, Sohn JC, Park CH, Kim CI. The usefulness of intravesical prostatic protrusion and bladder wall thickness measurement using transabdominal ultrasound in patients with benign prostatic hyperplasia. Korean J Urol. 2005. 46:1180–1185.
5. Nose H, Foo KT, Lim KB, Yokoyama T, Ozawa H, Kumon H. Accuracy of two noninvasive methods of diagnosing bladder outlet obstruction using ultrasonography: intravesical prostatic protrusion and velocity-flow video urodynamics. Urology. 2005. 65:493–497.
6. Lee JM, Chung H, Kim TW, Kim HS, Wang JH, Yang SK. The correlation of intravesical prostatic protrusion with storage symptoms, as measured by transrectal ultrasound. Korean J Urol. 2008. 49:145–149.
7. Doo CK, Uh HS. Anatomic configuration of prostate obtained by noninvasive ultrasonography can predict clinical voiding parameters for determining BOO in men with LUTS. Urology. 2009. 73:232–236.
8. John H, Hauri D, Bangerter U, Elbadawi A. Ultrastructure of the trigone and its functional implications. Urol Int. 2001. 67:264–271.
9. Kuo HC. Clinical prostate score for diagnosis of bladder outlet obstruction by prostate measurements and uroflowmetry. Urology. 1999. 54:90–96.
10. Lee SW, Cho JM, Kang JY, Yoo TK. Clinical and urodynamic significance of morphological differences in intravesical prostatic protrusion. Korean J Urol. 2010. 51:694–699.
11. Chia SJ, Heng CT, Chan SP, Foo KT. Correlation of intravesical prostatic protrusion with bladder outlet obstruction. BJU Int. 2003. 91:371–374.
12. Arnolds M, Oelke M. Positioning invasive versus noninvasive urodynamics in the assessment of bladder outlet obstruction. Curr Opin Urol. 2009. 19:55–62.
13. Madersbacher S, Alivizatos G, Nordling J, Sanz CR, Emberton M, de la Rosette JJ. EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines). Eur Urol. 2004. 46:547–554.
14. Franco G, De Nunzio C, Leonardo C, Tubaro A, Ciccariello M, De Dominicis C, et al. Ultrasound assessment of intravesical prostatic protrusion and detrusor wall thickness--new standards for noninvasive bladder outlet obstruction diagnosis? J Urol. 2010. 183:2270–2274.
15. Keqin Z, Zhishun X, Jing Z, Haixin W, Dongqing Z, Benkang S. Clinical significance of intravesical prostatic protrusion in patients with benign prostatic enlargement. Urology. 2007. 70:1096–1099.
16. Kim KH, Kim YS. Correlation of male overactive bladder with intravesical prostatic protrusion. Korean J Urol. 2010. 51:843–846.
17. Lee LS, Sim HG, Lim KB, Wang D, Foo KT. Intravesical prostatic protrusion predicts clinical progression of benign prostatic enlargement in patients receiving medical treatment. Int J Urol. 2010. 17:69–74.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download