Abstract
Purpose
This study examined the significance of tubulin α and βII expression in benign prostatic hyperplasia (BPH) and prostate cancer.
Materials and Methods
A total 103 cases diagnosed with either BPH or prostate cancer were divided into 4 groups: BPH (n=25), localized prostate cancer (n=41), locally advanced prostate cancer (n=18), metastatic prostate cancer (n=18). The tissues were taken through a transurethral resection of the prostate, suprapubic prostatectomy, prostatic needle biopsy or radical retropubic prostatectomy. The expression was measured by immunohistochemical staining. The degree of expression was graded on a 4-point scale. The data was compared in terms of stage, the serum prostatic specific antigen (PSA) level and Gleason score. The statistical comparisons were made using one-way ANOVA test and a Fisher's exact test.
Results
The levels of tubulin α and βII expression were stronger in the patients with prostate cancer than those with BPH (p<0.001). On the other hand, there was no difference between metastatic prostate cancer and localized prostate cancer (p>0.05). The level of tubulin α expression was stronger in the patients with a Gleason score ≥8 than in those of Gleason score ≤7 (p<0.05), but there was no such difference in tubulin βII expression. The level of tubulin α and βII expression was stronger in the patients with a serum PSA level ≥4ng/ml than in those with the serum PSA level <4ng/ml (p<0.001).
Figures and Tables
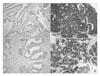 | Fig. 1Grading system in the expression of tubulin α and βII (immunohistochemical stain, ×100). The degree of expression was graded on a 4-point scale: 0 (negative stain), 1+ (focal stain), 2+ (multifocal stain), 3+ (diffuse stain). Negative stain for tubulin α in benign prostatic hyperplasia (BPH) tissue (A) and diffuse stain for tubulin α in metastatic prostate cancer (B). Negative stain for tubulin βII in BPH tissue (C) and diffuse stain for tubulin βII (D) in metastatic prostate cancer. |
References
1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006. 56:106–130.
2. Cancer Registration and Biostatistics Branch, National Cancer Center. Cancer Statistics in Korea. 2003.
3. Musch A. Microtubule organization and function in epithelial cells. Traffic. 2004. 5:1–9.
4. Sullivan KF, Cleveland DW. Identification of conserved isotype-defining variable region sequences for four vertebrate β-tubulin polypeptide classes. Proc Natl Acad Sci USA. 1986. 83:4327–4331.
5. Sullivan KF. Structure and utilization of tubulin isotypes. Annu Rev Cell Biol. 1988. 4:687–716.
6. Ludueña RF. Multiple forms of tubulin: different gene products and covalent modifications. Int Rev Cytol. 1998. 178:207–275.
7. Soucek K, Kamaid A, Phung AD, Kubala L, Bulinski JC, Harper RW, et al. Normal and prostate cancer cells display distinct molecular profiles of alpha-tubulin posttranslational modifications. Prostate. 2006. 66:954–965.
8. Hudes GR, Greenberg R, Krigel RL, Fox S, Scher R, Litwin S, et al. Phase II study of estramustine and vinblastin, two microtubule inhibitors, in hormone-refractory prostate cancer. J Clin Oncol. 1992. 10:1754–1761.
9. Pienta KJ, Redman B, Hussain M, Cummings G, Esper PS, Appel C, et al. Phase II evaluation of oral estramustine and oral etoposide in hormone-refractory adenocarcinoma of the prostate. J Clin Oncol. 1994. 12:2005–2012.
10. Hudes GR, Nathan FE, Khater C, Greenberg R, Gomella L, Stern C, et al. Paclitaxel plus estramustine in metastatic hormone-refractory prostate cancer. Semin Oncol. 1995. 22:5 Suppl 12. 41–45.
11. Lee JC, Harrison D, Timasheff SN. Interaction of vinblastine with calf brain microtubule protein. J Biol Chem. 1975. 250:9276–9282.
12. Rao S, Horwitz SB, Ringel I. Direct photoaffinity labeling of tubulin with taxol. J Natl Cancer Inst. 1992. 84:785–788.
13. Banerjee A, Roach MC, Trcka P, Luduena RF. Increased microtubule assembly in bovine brain tubulin lacking the type III isotype of β-tubulin. J Biol Chem. 1990. 265:1794–1799.
14. Banerjee A, Luduena RF. Distinct colchicine binding kinetics of bovine brain tubulin lacking the type III isotype of β-tubulin. J Biol Chem. 1991. 266:1689–1691.
15. Lu Q, Luduena RF. Removal of βIII isotype enhances taxol induced microtubule assembly. Cell Struct Funct. 1993. 18:173–182.
16. Panda D, Miller HP, Banerjee A, Luduena RF, Wilson L. Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proc Natl Acad Sci USA. 1994. 91:11358–11362.
17. Ranganathan S, Salazar H, Benetatos CA, Hedes GR. Immunohistochemical analysis of β-tubulin isotypes in human prostate carcinoma and benign prostatic hypertrophy. Prostate. 1997. 30:263–268.
18. McKean PG, Vaughan S, Gull K. The extended tubulin superfamily. J Cell Sci. 2001. 114:2723–2733.
19. Xu K, Ludueña RF. Characterization of nuclear βII-tubulin in tumor cells: a possible novel target for taxol. Cell Motil Cytoskeleton. 2002. 53:39–52.
20. Derry WB, Wilson L, Khan IA, Ludueña RF, Jordan MA. Taxol differentially modulates the dynamics of microtubules assembled from unfractionated and purified β-tubulin isotypes. Biochemistry. 1997. 36:3554–3562.
21. Sangrajrang S, Denoulet P, Laing NM, Tatoud R, Millot G, Calvo F, et al. Association of estramustine resistance in human prostatic carcinoma cells with modified patterns of tubulin expression. Biochem Pharmacol. 1998. 55:325–331.
22. Burkhart CA, Kavallaris M, Band Horwitz S. The role of beta-tubulin isotypes in resistance to antimitotic drugs. Biochim Biophys Acta. 2001. 1471:O1–O9.
23. Yeh IT, Ludueña RF. The βII isotype of tubulin is present in the cell nuclei of a variety of cancers. Cell Motil Cytoskeleton. 2004. 57:96–106.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download