Abstract
Purpose
At present, commercially available antiurolithic drugs have more adverse effects than potential therapeutic or preventive effects with chronic use. With this in mind, the present study was designed to assess the antiurolithic effect of olive oil in a mouse model of ethylene glycol (EG)-induced urolithiasis.
Materials and Methods
Adult albino mice were divided into 6 groups. Group I was fed the vehicle only. Group II was supplemented with 0.75% EG alone in drinking water during the experimental period to initiate deposition of calcium oxalate in kidneys, which leads to urolithiasis in animals. Groups III (olive oil control group) through V were fed olive oil orally at various doses during the experimental period. Group VI received cystone (750 mg/kg). Groups IV–VI additionally received 0.75% EG in drinking water ad libitum. SPSS ver.17.0 was used for statistical analysis.
Results
The study results showed significantly higher levels of serum urea, uric acid, and creatinine (p<0.05) in group II than in groups III–VI and I. Administration of olive oil at different doses restored the elevated serum parameters in groups IV and V compared with group II. Urine and kidney calcium, oxalate, and phosphate levels in groups IV–VI were significantly lower (p<0.05) than in animals with EG-induced urolithiasis (group II). Group V mice showed a significant restoration effect on serum as well as urine and kidney parameters compared with group II.
Nowadays, urinary calculi are an emerging, alarming global issue. The frequency of urinary calculi has increased owing to the dietary habits and lifestyle of the current generation as well as a rise in global warming. Urinary calculi affect the global population at a rate of around 12% [1]. Currently followed management procedures for the treatment of urinary stones include lithotripsy and surgical methods [2]. The recurrence rate seems to be high regardless of new advances in medical treatment management methods. Approximately 75% of these patients will have at least one recurrence [3] without proper treatment and preventive measures. Calcium phosphate is one of the significantly occurring urinary crystal sediments in most patients with urolithiasis [4]. Some studies have shown the presence of calcium phosphate in small amounts in patients with urethral and renal stone diseases [5]. It was also reported that a small amount of calcium phosphate is involved in stone attachment [6]. This evidence suggests that calcium phosphate plays a vital role in the formation of renal stones in the initial stages while the stones are attached to the renal papilla [7]. Worldwide, calcium oxalate (CaOx, CaC2O4) is the most predominant urinary calculi [1]. In the urinary tract, increased urinary supersaturation followed by the formation of solid crystalline particles is responsible for the beginning of crystallization of CaOx in the tract [8].
Despite extensive research in urology to date, there are as yet no effective, satisfactory drugs for dissolving or disintegrating urinary stones. Currently, most patients still rely on alternative medicine for better relief [9]. Therefore, alternative or complementary medicines with fewer side effects might be useful. Today's traditional herbal medicines aid in the development of potential therapeutic drugs in the form of either extracts alone, extracts in combination with other herbs, or drugs in the form of phytochemical compounds isolated from herbs.
Since the ancient era, the olive tree (Olea europaea) has been known for its medicinal properties in the Mediterranean region. Some studies have reported that the leaves and extracts of olive trees have nutritional importance and can be used as alternative remedies for numerous illnesses [10]. In the Mediterranean basin region, the use of olive oil in traditional medicine for several thousands of years is associated with a low occurrence of heart disease and some types of cancer [11]. Olive oil is well known for its hypotensive, antioxidant, cardiovascular, hypoglycemic, and hepatoprotective effects [1213141516]. Reports on the beneficial effect of the Mediterranean diet for nephrolithiasis are rare, however [17]. There is no previous literature regarding the antiurolithic activity of olive oil in vivo. With this in mind, the current study aimed to explore the antiurolithic activity of olive oil in an ethylene glycol (EG)-induced urolithiasis model and the role of olive oil in the prevention of renal stones.
All experimental research analytical-grade reagents and chemicals were procured from Sigma Chemical Co. Inc. (St. Louis, MO, USA). All bioassay kits (uric acid, urea, creatinine, oxalate, inorganic phosphate, and calcium) were purchased from Biovision Inc. (Milpitas, CA, USA). Olive oil (extra virgin) was purchased from Sakaka olive market (Al-Jawf, Saudi Arabia).
To determine the suitable safe olive oil dose, acute toxicity was assessed in mice according to the Organisation for Economic Co-operation and Development protocol [18]. Briefly, healthy normal mice were grouped into 4 cages of 5 animals each. Oral doses of 0.5, 0.75, 1.0, 1.3, and 1.7 mL/kg body weight of olive oil was supplemented to each group with ad libitum access to water and food. The animals were observed for general behavior and signs of toxicity for 1 hour, 4 hours, and over the period of 24 hours after drug administration. The animals were further observed for signs of toxicity, weight loss, and mortality for 14 days.
Adult male mice (Swiss albino) weighing 20 to 25 g were obtained from the medical school animal husbandry department at Al Jouf University. The college ethical committee approved the protocols to conduct this study. The experimental animals were kept in 12-hour dark-light cycles at 25℃ with ad libitum access to food and water. Mice were divided into 6 groups with 10 mice in each group. Group I consisted of normal mice supplemented with vehicle only. Group II mice received 0.75% EG alone in drinking water. The olive oil-supplemented normal controls (group III) received standard pellets + oral olive oil (1.3 mL/kg body weight). Groups IV and V were fed orally with olive oil at various doses along with EG (0.75%) in drinking water for 28 days. Group VI received cystone (750 mg/kg) as a standard drug and 0.75% EG in drinking water during the experimental period (28 days) [19]. EG (0.75% in drinking water) was used in groups IV through VI to induce urolithiasis and to promote the development of calcium oxalate crystals in the kidney during the experimental period [20].
At the end of the experiment (on day 28), all animals were kept in metabolic cages individually for the collection of 24-hour urine samples. 3N HCl was added to the urine samples, followed by a 10-minute centrifugation at 1,500 rpm. The supernatant and debris were discarded and the samples were kept at -20℃. A Chemwell Biochemistry automatic analyzer (Awareness Technology, Inc., Palm City, FL, USA) was used for oxalate, calcium, and inorganic phosphate measurement in urine.
After they had been deprived of food for 12 hours overnight, the mice were anesthetized with diethyl ether. After cervical decapitation, blood samples were collected into nonanticoagulant-treated test tubes and allowed to clot at room temperature. Serum samples were obtained by centrifugation for 15 minutes at 1,500 rpm. The samples were subjected to serum urea, creatinine, and uric acid analysis with the aid of commercially available bioassay kits according to the manufacturer's protocols by use of a Chemwell Biochemistry automatic analyzer.
Both kidney samples were collected immediately after the animals were sacrificed under anesthesia, followed by rinsing in cold 0.15 M KCl. After that, the right kidney was minced with scissors and then homogenized with 0.15 M KCl by use of a Remi's glass homogenizer. The supernatant sample was obtained from the homogenate by centrifugation for 15 minutes at 1,500 rpm. The supernatant was used for estimation of oxalates by use of commercially available bioassay kits according to the manufacturer's protocols by use of the Chemwell Biochemistry automatic analyzer.
The study parameters were conducted in triplicate. Mean±standard deviation was used to express the data. To compare the groups, one-way analysis of variance was applied with the aid of SPSS ver. 17.0 (SPSS Inc., London, United Kingdom). Statistical significance was considered as p<0.05.
The study results showed that olive oil at doses of 1.3 and 1.7 mL/kg body weight orally was suitable for supplementation of the experimental animals. No toxic symptoms or abnormal behavior were observed in the mice with these doses.
Our results showed that serum urea, uric acid, and creatinine were significantly (p<0.05) higher in group II animals than in group I (Table 1). However, olive oil treatment (1.3 and 1.7 mL/kg body weight orally) significantly (p<0.05) lowered serum uric acid, creatinine, and urea levels in animals in groups IV and V compared with group II. Group VI animals showed significantly (p<0.05) lower levels of serum urea, uric acid, and creatinine than animals in groups II, IV, and V. By contrast, group V animals (1.7 mL/kg body weight orally) showed significantly lower levels of serum urea, uric acid, and creatinine than did animals in group IV (1.3 mL/kg body weight orally), with a resultant significantly (p<0.05) greater decease compared with group II in serum urea, uric acid, and creatinine in group V than in group IV.
The study results revealed significantly (p<0.05) higher levels of urinary oxalate, calcium, and inorganic phosphate in group II than in the other groups (Table 2). However, supplementation with olive oil in groups IV and V resulted in significantly (p<0.05) decreased levels of oxalate, calcium, and inorganic phosphate in urine compared with group II. Cystone-treated animals (group VI) showed significantly (p<0.05) lower levels of urinary oxalate, calcium, and phosphate than in group II animals (Fig. 1). In contrast, a similar type of effect was observed in group IV and V animals after administration of olive oil (1.3, 1.7 mL/kg body weight orally) in a dose-dependent manner.
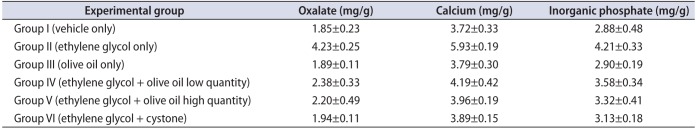
The kidney homogenate showed significantly (p<0.05) higher levels of oxalate, calcium, and inorganic phosphate in group II animals than in the other groups (Table 3). However, administration of olive oil to the animals in groups IV and V resulted in significantly lower (p<0.05) levels of oxalate, calcium, and inorganic phosphate in kidney homogenate compared with the EG-induced urolithiasis animal group (group II). Cystone-treated animals (group VI) exhibited significantly (p<0.05) lower levels of kidney oxalate, calcium, and phosphate than did group II animals (Fig. 2). In groups IV and V, the elevated levels of kidney homogenate parameters such as oxalate, calcium, and phosphate were restored after supplementation with olive oil (1.3 and 1.7 mL/kg body weight orally).
Nowadays, urinary stones have an important place in urological practice. Men predominate over women in risk of stone formation, with an incidence in the range of 5% to 10% that is observed to peak between the ages of 40 and 50 years. The recurrence of stone formation is very common in patients with renal stone disease. Based on the constituents (calcium) of the stone, renal stones are classified into calcium oxalate dehydrate, calcium oxalate monohydrate, and basic calcium phosphate stones, which commonly occur at a rate of 75% to 90%, followed by magnesium ammonium phosphate (struvite), uric acid, and cystine stones, respectively (10%–15%, 3%–10%, and 0.5%–1%, respectively). Generally, magnesium ammonium phosphate or calcium oxalate stones are more common among all types of stones. Many medications and alternative remedies have been used to treat urinary stones in the past several years.
Generally, the majority of practice in traditional medicine is based on plants and their products, but the rationale behind their use is often not scientifically well established through clinical trials and systematic pharmacological studies. Only a few herbal drugs have supportive scientific evidence for their potential activity. However, the use of plant products in traditional medicine for renal stone therapy has assumed importance.
Previous studies showed that flavonoids may reduce renal apoptosis owing to their antioxidant and diuretic properties. Use of synthetic drugs in the treatment of urolithiasis on a long-term basis could lead to adverse effects. Extracorporeal shock wave lithotripsy is the standard procedure for the removal of renal stones in current renal stone management compared with open renal surgery [21].
The potential role of plant extracts in the prevention of kidney stone formation is through inhibition of the growth of CaOX crystals induced by urinary macromolecules and excretion of the CaOx crystals attached to the surface of the epithelial cells along with the urine [22]. EG-induced renal calcium oxalate in mice is used to mimic the formation of urinary stones in humans [23]. Therefore, in the present study, the EG-induced urolithiasis model was used to access the anti-urolithiasis effect of olive oil.
In urolithiasis, obstruction of the urinary system due to the formation of calculi in the renal tissue leads to a decrease in the kidney glomerular filtration rate, which results in the accumulation of nitrogenous waste produces such as uric acid, serum urea nitrogen, and creatinine in the blood [24]. In this study, elevated serum urea, uric acid, and creatinine levels were seen in mice with EG-induced urolithiasis, with marked renal damage that may have been due to the release of nitrogenous waste substances in the blood. However, curative treatment with olive oil (1.3, 1.7 mL/kg body weight orally) may causes dieresis and accelerate the preformed stone-dissolving process and also may prevent the formation of new renal stones in the urinary system.
In the present study, hyperoxaluria resulted after the administration of 0.75% EG in group II animals [25]. A biochemical mechanism is associated with elevated concentrations of oxalate in the urine of group II animals administered the 0.75% EG solution. Hyperoxaluria results from increased retention and excretion of oxalate from kidneys [26]. The urinary excretion of oxalate in hyperoxaluria is comparatively more important in the formation of urinary stones than the urinary excretion of calcium [27]. Hyperoxaluria leads to the formation of calcium oxalate or apatite from the urine and the resultant development of crystals [27]. It has been reported that hyperabsorption of calcium is due to defective renal tubular reabsorption [28]. Hyperoxaluria can cause the formation of renal stones in EG-fed animals owing to increased renal oxalate retention and excretion [29]. Our study results show that administration of olive oil (1.3, 1.7 mL/kg body weight orally) restored oxalate, inorganic phosphate, and calcium levels in urine similar to those in group VI (cystone-treated animals).
EG-induced toxicity targets the kidney. Generally, in hyperoxaluria, EG is metabolized to four main organic acids in vivo, glycooxalic acid, glycolaldehyde, glycolic acid, and oxalic acid, which is the initiative factor for lithiasis [30]. Hence, in this study, EG was preferred for use to develop urolithiasis. The elevated levels of calcium, oxalate, and phosphate in the kidney homogenate supernatant of droups IV and V were also restored with olive oil supplementation in this study compared with the EG control group.
The study results indicate that administration of olive oil (1.7 mL/kg body weight) reduced and prevented the growth of urinary stones. The current study revealed the antiurolithic effect of olive oil (1.7 mL/kg body weight) in an EG-induced renal calculi model. Therefore, olive oil (1.7 mL/kg body weight) may prevent renal stone formation by inhibiting damage to the renal tubular membrane due to peroxidative stress induced by hyperoxaluria. The underlying biochemical mechanism of this potential effect of olive oil may be its antioxidant properties. However, more investigation is required to clarify the exact mechanism of this action.
ACKNOWLEDGMENTS
This work was made possible by the financial support of Al Jouf University (Research project No. 312/35), Saudi Arabia.
References
1. Trinchieri A. Epidemiological trends in urolithiasis: impact on our health care systems. Urol Res. 2006; 34:151–156. PMID: 16440192.

2. Christina AJ, Priya Mole M, Moorthy P. Studies on the antilithic effect of Rotula aquatica lour in male Wistar rats. Methods Find Exp Clin Pharmacol. 2002; 24:357–359. PMID: 12224442.

3. Asplin J, DeGanello S, Nakagawa YN, Coe FL. Evidence that nephrocalcin and urine inhibit nucleation of calcium oxalate monohydrate crystals. Am J Physiol. 1991; 261(5 Pt 2):F824–F830. PMID: 1951713.

4. Fan J, Chandhoke PS. Examination of crystalluria in freshly voided urines of recurrent calcium stone formers and normal individuals using a new filter technique. J Urol. 1999; 161:1685–1688. PMID: 10210440.

5. Grases F, March JG, Conte A, Costa-Bauzá A. New aspects on the composition, structure and origin of calcium oxalate monohydrate calculi. Eur Urol. 1993; 24:381–386. PMID: 8262107.
6. Miller NL, Evan AP, Lingeman JE. Pathogenesis of renal calculi. Urol Clin North Am. 2007; 34:295–313. PMID: 17678981.

7. Coe FL, Evan AP, Lingeman JE, Worcester EM. Plaque and deposits in nine human stone diseases. Urol Res. 2010; 38:239–247. PMID: 20625890.

8. Basavaraj DR, Biyani CS, Browning AJ, Cartledge JJ. The role of urinary kidney stone inhibitors and promoters in the pathogenesis of calcium containing renal stones. EAU-EBU Update Ser. 2007; 5:126–136.

9. Cheesman EE. Classification of the bananas. the genus Ensete Horan. Kew Bulletin. 1968; 2:97–106.

10. Flynn MM, Reinert SE. Comparing an olive oil-enriched diet to a standard lower-fat diet for weight loss in breast cancer survivors: a pilot study. J Womens Health (Larchmt). 2010; 19:1155–1161. PMID: 20545561.

11. Hu FB. The Mediterranean diet and mortality--olive oil and beyond. N Engl J Med. 2003; 348:2595–2596. PMID: 12826632.
12. Benavente-García O, Castillo J, Lorente J, Alcaraz M. Radioprotective effects in vivo of phenolics extracted from Olea europaea L. leaves against X-ray-induced chromosomal damage: comparative study versus several flavonoids and sulfur-containing compounds. J Med Food. 2002; 5:125–135. PMID: 12495584.
13. Khayyal MT, el-Ghazaly MA, Abdallah DM, Nassar NN, Okpanyi SN, Kreuter MH. Blood pressure lowering effect of an olive leaf extract (Olea europaea) in L-NAME induced hypertension in rats. Arzneimittelforschung. 2002; 52:797–802. PMID: 12489249.
14. Komaki E, Yamaguchi S, Maru I, Kinoshita M, Kakehi K, Ohta Y, et al. Identification of anti-alpha-amylase components from olive leaf extracts. Food Sci Technol Res. 2003; 9:35–39.

15. Somova LI, Shode FO, Mipando M. Cardiotonic and antidysrhythmic effects of oleanolic and ursolic acids, methyl maslinate and uvaol. Phytomedicine. 2004; 11:121–129. PMID: 15070161.

16. Poudyal H, Campbell F, Brown L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate-, high fat-fed rats. J Nutr. 2010; 140:946–953. PMID: 20335636.

17. Soldati L, Bertoli S, Terranegra A, Brasacchio C, Mingione A, Dogliotti E, et al. Relevance of Mediterranean diet and glucose metabolism for nephrolithiasis in obese subjects. J Transl Med. 2014; 12:34. PMID: 24502605.

18. OECD. OECD guidelines for acute toxicity of chemicals: acute oral toxicity – fixed dose procedure. OECD code 420. Paris (France): Organisation for Economic Co-operation and Development;2001.
19. Mitra SK, Gopumadhavan S, Venkataranganna MV, Sundaram R. Effect of cystone, a herbal formulation, on glycolic acid-induced urolithiasis in rats. Phytother Res. 1998; 12:372–374.

20. Christina AJ, Najumadeen NA, Kumar SV. Antilithiatic effect of Melia azedarach. on ethylene glycol-induced nephrolithiasis in rats. Pharm Biol. 2006; 44:480–485.
21. Tripathi KD. Relevant physiology of urine formation; essentials of medical pharmacology. Arch Ital Urol Androl. 2005; 77:557–560.
22. Kishimoto T, Yamamoto K, Sugimoto T, Yoshihara H, Maekawa M. Side effects of extracorporeal shock-wave exposure in patients treated by extracorporeal shock-wave lithotripsy for upper urinary tract stone. Eur Urol. 1986; 12:308–313. PMID: 3780794.
23. Bashir S, Gilani AH. Antiurolithic effect of Bergenia ligulata rhizome: an explanation of the underlying mechanisms. J Ethnopharmacol. 2009; 122:106–116. PMID: 19118615.

24. Nayeem K, Gupta D, Nayana H, Joshi RK. Antiurolithiatic potential of the fruit extracts of Carica papaya on ethylene glycol induced urolithiatic rats. J Pharm Res. 2010; 3:2772–2775.
25. Ghodkar PB. Chemical tests in kidney disease. In : Godkar PB, Godkar DP, editors. Textbook of medical laboratory technology. Mumbai: Bhalani Publishing House;1994. p. 118–132.
26. Atmani F, Slimani Y, Mimouni M, Hacht B. Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. BJU Int. 2003; 92:137–140. PMID: 12823398.

27. Robertson WG, Peacock M. The cause of idiopathic calcium stone disease: hypercalciuria or hyperoxaluria? Nephron. 1980; 26:105–110. PMID: 7412965.

28. Lemann J Jr, Worcester EM, Gray RW. Hypercalciuria and stones. Am J Kidney Dis. 1991; 17:386–391. PMID: 2008904.

29. Fossati P, Prencipe L, Berti G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem. 1980; 26:227–231. PMID: 7353268.

30. Afonne OJ, Orisakwe OE, Dioka CE, Obi E, Ezejiofor T, Asomugha L, et al. Effects of rinbacin extract on rat kidney. Biol Pharm Bull. 2002; 25:1022–1025. PMID: 12186402.

Fig. 1
(A) Effect of olive oil on urine calcium levels in mice with ethylene glycol-induced urolithiasis. (B) Effect of olive oil on urine oxalate levels in mice with ethylene glycol-induced urolithiasis. (C) Effect of olive oil on urine inorganic phosphate levels in mice with ethylene glycol-induced urolithiasis. Group I, vehicle only; group II, ethylene glycol only; group III, olive oil only; group IV, ethylene glycol + olive oil low quantity; group V, ethylene glycol + olive oil high quantity; group VI, ethylene glycol + cystone.

Fig. 2
(A) Effect of olive oil on kidney oxalate levels in mice with ethylene glycol-induced urolithiasis. (B) Effect of olive oil on kidney calcium levels in mice with ethylene glycol-induced urolithiasis. (C) Effect of olive oil on kidney inorganic phosphate levels in mice with ethylene glycol-induced urolithiasis. Group I, vehicle only; group II, ethylene glycol only; group III, olive oil only; group IV, ethylene glycol + olive oil low quantity; group V, ethylene glycol + olive oil high quantity; group VI, ethylene glycol + cystone.

Table 1
Effect of olive oil on serum parameters in mice with ethylene glycol-induced urolithiasis
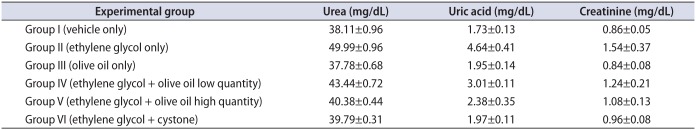
Table 2
Effect of olive oil on urine parameters in mice with ethylene glycol-induced urolithiasis
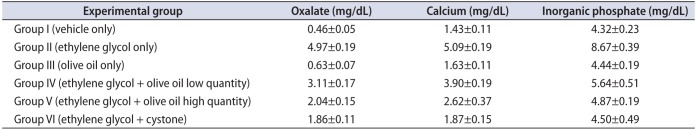
Table 3
Effect of olive oil on kidney parameters in mice with ethylene glycol-induced urolithiasis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download