INTRODUCTION
Acute lymphocytic leukemia (ALL) is a rapidly progressive malignant neoplasm of the hematopoietic system (
1). A diagnosis of acute leukemia is based on the presence of large numbers of blast cells in either peripheral blood or bone marrow with concurrent cytopenia. Malignancy can be diagnosed by blast cell morphology (medium to large immature cells, with round or indented nuclei containing fine stippled chromatin), with or without CD34
+ staining of precursor cells. Immunophenotyping by flow cytometry can allow classification of lymphoid cells as B- or T-cell in origin, and B-cell malignancy is predominant in canine ALL (
23). PCR for antigen receptor rearrangement (PARR) can also provide neoplastic cell detection and immunophenotyping, but its use in cases of ALL in dogs has been limited.
Here we report a dog with ALL initially diagnosed using cytology. PARR could detect malignancy and T-cell origin, but immunophenotypic analysis by flow cytometry analysis using peripheral blood cells showed limited information due to a blood transfusion and severe leukopenia.
MATERIALS AND METHODS
A 14-year-old spayed female mixed-breed dog was admitted to a local veterinary clinic for severe anemia. It was an indoor dog, and tick-borne diseases were excluded using the IDEXX SNAP
® 4Dx kit (IDEXX Laboratories, Westbrook, ME, USA). The local veterinary clinic found severe anemia (hematocrit: 16.3%, reference interval: 37%–55%), remarkable monocytosis (69.94%; 168.79×10
3/µL, reference interval: 3%–14%, 0.2–2×10
3/µL, respectively), and thrombocytopenia (65×10
3/µL, reference interval: 200–500×10
3/µL) on an automated impedance cell counter (Hemavet 850; CDC Technologies, Oxford, CT, USA). Unstained blood film was referred to the veterinary medical teaching hospital (VMTH) for detailed cytological evaluation. On light microscopic examination of the blood smear, immature lymphoid cells featuring a large nucleus with small amounts of cytoplasm, prominent nucleoli, dispersed chromatin, and dark cytoplasm were predominant (
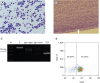
Fig. 1A). ALL was suggested based on microscopic examination of the blood film.
Figure 1
(A) Microscopic examination of peripheral blood prior to administration. (B) Microscopic examination of feathered edge of blood film on admission. (C) PCR for analysis of antigen receptor rearrangement from peripheral blood. First lane ‘M’ shows a 25 bp size marker, lane ‘C’ indicates positive control for DNA, lane 3 and 4 detect “major” and “minor” immunoglobulin rearrangements, respectively, and lane 5 (TCR) detects TCRγ gene rearrangement. (D) A dot plot from flow cytometry analysis. Immature lymphoid cells were prominent prior to administration but microscopic examination was not easy because of severe leukopenia on admission. PARR revealed positive for clonal rearrangement of TCRγ gene but flow cytometry did not show overt T cell predominance after blood transfusion in this dog.
TCR, T-cell receptor; PARR, PCR for antigen receptor rearrangement.
Ten days later, the dog was admitted to the VMTH for a medical emergency. The client noticed lethargy and anorexia, and the local veterinarian transferred the dog due to pale mucous membranes and weak pulse. On physical examination, the submandibular lymph nodes were firm, but superficial lymphadenopathy, cutaneous masses, and ulcers were not observed. Complete blood counts (CBCs) by laser cytometry (Procyte; IDEXX Laboratories) revealed remarkable leukopenia, severe non-regenerative anemia, and severe thrombocytopenia (
Table 1). Lymphoblast leukemia was strongly suspected, but neither microscopic confirmation of predominant lymphoblast nor flow cytometry analysis could be performed due to severe leukopenia (
Fig. 1B). ALL was tentatively diagnosed based on prior blood film examination, and an emergency transfusion of fresh whole blood was performed.
Table 1
Hematological profile on the day of presentation

|
Parameters |
On admission |
Reference intervals |
|
Red blood cell count (×1012/L) |
1.98 |
5.65–8.87 |
|
Hemoglobin (g/dL) |
4.00 |
13.10–20.50 |
|
Hematocrit (%) |
10.60 |
37.30–61.70 |
|
Mean cell volume (fL) |
53.50 |
61.60–73.50 |
|
Mean cell hemoglobin concentration (g/L) |
377.00 |
320.00–379.00 |
|
Reticulocyte count (×109/L) |
2.60 |
10.00–110.00 |
|
White blood cell count (×109/L) |
2.11 |
5.05–16.76 |
|
Neutrophil count (×109/L) |
0.08 |
2.95–11.64 |
|
Lymphocyte count (×109/L) |
0.87 |
1.05–5.10 |
|
Monocyte count (×109/L) |
1.16 |
0.16–1.12 |
|
Eosinophil count (×109/L) |
0 |
0.06–1.23 |
|
Platelet count (×109/L) |
3.00 |
148.00–484.00 |
For further confirmative diagnostic evaluation, PARR from peripheral blood was performed as reported previously with minor modifications (
4). Briefly, DNA was extracted from whole blood cells using a commercially available kit (QIAamp
® DNA Mini Kit; QIAGEN GmbH, Hilden, Germany). Amplification of immunoglobulin heavy chain CDR3 regions and T-cell receptor gamma (TCRγ) sequences was performed using previously described primers (
56). A PCR premixture (LeGene Biosciences, San Diego, CA, USA) was used for DNA amplification, and amplicons were visualized using polyacrylamide gel electrophoresis techniques. Expected amplicons size are 130 bp for Cµ (DNA control), 120 bp for immunoglobulin products and 90 bp for TCR products.
Flow cytometry analysis was performed the day after the transfusion. CD21 (B cells, clone CD2.1D6), CD3 (all T cells, clone CA17.2A12), CD4 (T cell subsets, clone YKIX302.9), and CD8 (T cell subsets, clone YCATE55.9) antibodies (Bio-Rad Laboratories, Hercules, CA, USA) were incubated with 200 µL of whole blood for 30 min at room temperature; fixing and red blood cell lysis were performed using FACS™ Lysing Solution (Becton, Dickinson and Co., Franklin Lakes, NJ, USA).
A clonal rearrangement of the TCRγ gene was confirmed by PARR (
Fig. 1C). However, the flow cytometry test for T-cell lymphoid neoplasia was negative; only 70.03% of lymphocytes were positive for CD3 (
Fig. 1D). ALL was confirmed based on microscopic examination and PARR, for which specificity was high. We have concluded blood transfusion might mask cellular composition of lymphocyte subsets in flow cytometry analysis. We informed the owner that the prognosis of ALL is usually poor, and the owner elected to pursue only supportive therapy. The dog's condition continued to deteriorate, and died 1 wk after diagnosis. The client refused bone marrow examination and necropsy.
In this case, flow cytometry was not a reliable diagnostic method for an ALL dog after blood transfusion, whereas PARR could detect lymphoid malignancy. Our results suggest that PARR should be the first-line diagnostic tool in dogs that have recently received blood transfusions.
RESULTS AND DISCUSSION
Canine ALL is a hematopoietic neoplasm and cytopenia are a common feature (
7). The clinical signs might be related to cytopenia but not specific even if it is rapid and progressive. Since ALL is usually poorly responsive to therapy and has poor prognosis, immediate diagnosis can help owners to understand the disease process. Initially, the owner observed lethargy and anorexia; non-regenerative anemia was the remarkable laboratory finding in this dog. Anemia may involve myelophthisis, erythropoiesis inhibition, be secondary immune-mediated, or involve hemorrhage secondary to platelet deficiency (
8). Blood transfusion may not be recommended as a long-term management plan because of poor prognosis, but the owner requested it as emergency care. Clinicians should be aware of possible diagnostic errors when blood transfusion is performed before a definitive diagnosis is reached. Both leukocytosis and leukopenia may be observed in ALL, but neutropenia is much more common (
23). In this case, leukocytosis was initially observed at the private clinic, but acute leukopenia developed, making diagnosis based on cytology and flow cytometry difficult. Possible mechanisms of neutropenia are bone marrow infiltrate, action of inhibitory factors on myelopoiesis, or immune-mediated destruction (
2).
There are several diagnostic tools for ALL: microscopic examination, PARR, and flow cytometry analysis. Malignancy can be diagnosed by microscopic examination alone, but PARR can help detect clonal rearrangement of neoplastic cells. Both PARR and flow cytometry can provide immunophenotyping. However, there are limitations to ALL diagnosis among these diagnostic tools. Morphology cannot always distinguish leukemia of lymphoid or myeloid origin. Low cell numbers in peripheral blood make it difficult to recognize a hematopoietic neoplasm with microscopic examination and flow cytometry analysis. PARR has high specificity, but its sensitivity varies (
9). In this case, we diagnosed ALL using microscopic examination and PARR. However, flow cytometry analysis was difficult due to severe leukopenia. Proper leukocyte numbers are essential for evaluation of the lymphocyte populations. Moreover, leukocyte contamination from donor blood after transfusion could mask the patient's cell population. However, PARR is very sensitive and can detect one neoplastic lymphoid cell per 100 cells (
5). PARR is useful when flow cytometry is not possible, such as for analysis of samples from body cavity fluid. In addition, the principle of PARR is detection of lymphoid malignancy, whereas flow cytometry screens cell populations without detecting malignancy. Thus, microscopic examination is essential to identify cellular malignancy when flow cytometry is used. In this case, ALL was tentatively diagnosed by microscopic examination when the dog was not leukopenic, and additional in-house PARR was very useful for confirmation of ALL. However, the lymphocyte population was too low to confirm morphologic evaluation, as leukopenia was present at the VMTH. Low leukocyte numbers made flow cytometry analysis difficult, as well, and this diagnostic challenge was also present after blood transfusion. Moreover, in rare cases of acute leukemia, the precursor cells can be very small and lack overt nucleoli (
1). PARR should be the first-choice diagnostic test in dogs with leukopenia or for samples with low cell numbers. Meanwhile, clinicians should be aware that PARR has variable sensitivity and specificity based on the laboratory that performs the test (
9); thus, clinical examination and cytological evaluation should also be performed concurrently.
Differentiation of ALL from stage V multicentric lymphoma is not easy. ALL, however, has more rapid progression, lacks significant lymphadenopathy, responds poorly to chemotherapy, and has a CD34
+ progenitor cell immunophenotype. Lymphoma typically shows prominent lymphadenopathy, whereas the primary sites of involvement in leukemia are the bone marrow and spleen; thus, lymphadenopathy predominates in lymphoma (
2). The presence of the CD34 surface antigen can differentiate between the neoplastic lymphoid cells of ALL and stage V lymphoma; however, it has been reported that approximately 75% of clinically defined cases of canine ALL expressed CD34 (
21011). Immunophenotyping with CD34 could help differentiate ALL from lymphoma, but physical examination, cytology, hematologic data, and PARR were more useful in this case.
Immunophenotyping of chronic lymphoproliferative leukemia is T-cell predominant, whereas a high prevalence of B cells indicates acute lymphoproliferative disorder (
23). We diagnosed T-cell ALL in this case, and the survival time was very short. As the clinical significance of cell origin in dogs with ALL or chronic lymphocytic leukemia is unknown, larger canine studies to develop prognostic biomarkers of genetic mutations in
Ras,
FLT3, and
C-kit are necessary.
In conclusion, we report ALL in a dog diagnosed using PARR. A blood transfusion might mask microscopic examination of blood film and flow cytometry analysis. Clinicians should be aware that PARR is a more reliable diagnostic tool than flow cytometry analysis when a dog has received a blood transfusion.



