Abstract
Background
Insulin resistance is an integral feature of metabolic syndromes, including obesity, hyperglycemia, and hyperlipidemia. In this study, we evaluated whether the aloe component could reduce obesity-induced inflammation and the occurrence of metabolic disorders such as blood glucose and insulin resistance.
Methods
Male C57BL/6 obese mice fed a high-fat diet for 54 days received a supplement of aloe formula (PAG, ALS, Aloe QDM, and Aloe QDM complex) or pioglitazone (PGZ) and were compared with unsupplemented controls (high-fat diet; HFD) or mice fed a regular diet (RD). RT-PCR and western blot analysis were used to quantify the expression of obesity-induced inflammation.
Results
Aloe QDM lowered fasting blood glucose and plasma insulin compared with HFD. Obesity-induced inflammatory cytokine (IL-1β, -6, -12, TNF-α) and chemokine (CX3CL1, CCL5) mRNA and protein were decreased markedly, as was macrophage infiltration and hepatic triglycerides by Aloe QDM. At the same time, Aloe QDM decreased the mRNA and protein of PPARγ/LXRα and 11β-HSD1 both in the liver and WAT.
Conclusion
Dietary aloe formula reduces obesity-induced glucose tolerance not only by suppressing inflammatory responses but also by inducing anti-inflammatory cytokines in the WAT and liver, both of which are important peripheral tissues affecting insulin resistance. The effect of Aloe QDM complex in the WAT and liver are related to its dual action on PPARγ and 11β-HSD1 expression and its use as a nutritional intervention against T2D and obesity-related inflammation is suggested.
Insulin resistance is defined as a decrease of the peripheral tissues to insulin action. Individuals which insulin resistance are predisposed to developing type-2 diabetes (T2D). Also, insulin resistance has been recognized as an integral feature of various metabolic syndromes including obesity, hyperglycemia, and hyperlipidemia.
In recent years, a large number of human population studies have linked insulin resistance to systemic inflammation (1,2) and have indicated that obesity-induced inflammation plays a crucial role in the development of metabolic disease (3,4). Inflammatory responses initiate obesity in adipose tissue. It is now well understood that adipose tissue is not simply a storage depot for excess calories but that it also actively secrets fatty acids and a variety of cytokines. Adipocytes uniquely secret adipokines, such as leptin and adionectin (5), that promote insulin sensitivity, as well as proteins, such as resistin (6), and retinol-binding protein 4 (RBP4) (7), which impair insulin sensitivity. Dysregulation of adipose tissue-derived proteins such as cytokines/chemokines and adipokines has been shown to result in impaired insulin signaling and lipid metabolism (8,9). Adipose tissue from obese individuals has been shown to be infiltrated with increased numbers of macrophages, a finding which provided a major mechanistic advance into the understanding of how obesity propagates inflammation.
Adipose tissue macrophages (ATM) are major source of pro-inflammatory cytokines. Activation of these tissue macrophages has been shown to lead to the release of a variety of chemokines, which in turn recruit additional macrophages, setting up a feed forward process that further increases ATM content and propagates the chronic inflammatory state (10). For example, the proinflammatory cytokine, TNF-α, has been demonstrated to mediate insulin resistance as a result of obesity in many rodent obesity models (11). TNF-α was over-expressed in white adipose tissue (WAT) in obese and insulin resistance state; mice lacking the TNF-α ligand or the TNF-α receptor were partially protected from obesity-induced insulin resistance (12).
Moreover, adipose tissue-derived factors including free fatty acids have been demonstrated to alter hepatic metabolism via their paracrine action, leading to abnormal fat accumulation and hepatic insulin resistance (13). It also has been shown that TNF-α and IL-6 are key mediators of hepatic inflammation and liver cell death, indicating that metabolic dysregulation may be aggravated systemically by these adipose tissue-derived factors. In this regard, reducing obesity-induced inflammation by targeting adipose tissue-derived proteins may be a useful strategy for preventing obesity-induced metabolic pathologies.
One of the most effective of the currently available medications for T2D is the thiazolidione (TZDs) class of insulin-sensitizing drugs. These TZDs function in binding to the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ). In WAT, PPARγ activation promotes adipogenesis and the differentiation of new adipocytes. Despite increasing total adipose tissue mass, TZDs have been suggested to improve systemic insulin sensitive cells and increase the production of adiponectin, a glucose-sensitizing peptide that holds anti-inflammatory properties (14). In addition, PPARγ ligands have been shown to markedly reduce the production of proinflammatory cytokine secretion in WAT, an effect that has been associated with a significant reduction in macrophage infiltration.
Aloe species have been used for centuries for their laxative, antiinflammatory, immunostimulant, antiseptic (15), burn healing (16), antiulcer (17), and antitumour (18) activities. In the past 15 years, there have also been reports regarding the antidiabetic activity of Aloe extracts (19,20).
Fifty years ago, Mertz and coworkers proposed that chromium (Cr) was an essential trace element (21) and that is was required for normal carbohydrate and lipid metabolism (22). Signs of chromium deficiency have been documented on numerous occasions, including elevated blood glucose and decreased high density lipoproteins (HDL) in humans with normal diets. Herein, we used a diet-induced obesity (DIO) mouse, an animal model of T2D, to examine whether the administration of Aloe vera with Cr could minimize the effects of hypoglycemia.
Our experiments revealed that Aloe QDM reduced obesity-induced glucose tolerance and insulin resistance by modulating inflammatory response both in liver and WAT. Hence, Aloe QDM complex may be useful as a dietary adjuvant for reducing obesity-induced metabolic disorders.
Processed Aloe vera gel (PAG) (23), Aloesin (ALS), Aloe QDM, and Aloe QDM complex (24) were provided by Univera, Inc. (Seoul, Republic of Korea). Pioglitazone (Actos®) was purchased from Eli Lilly (Toronto, Canada); chromium (Cr) was purchased from Lallemand Inc. (Montreal, Canada); and leupeptin, aprotinin, and phenylmethylsulfonyl fluoride (PMSF) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Antibodies of inducible nitric oxide synthase (i-NOS), Cyclooxygenase-2 (COX-2), Interleukin (IL)-1 beta (IL-1β), Interleukin (IL)-6, and Tumor necrosis factor (TNF)-alpha (TNF-α) were purchased from Santa Cruz Biotechnology Inc., (Santa Cruz, CA, USA). Anti-mouse interleukin (IL)-12, Interleukin (IL)-4 and Interleukin (IL)-10 were purchased from BD Biosciences (San Jose, CA, USA). Anti-mouse chemokine (C-X3-C motif) ligand 1 (CX3CL1) and ATP-binding cassette, sub-family A (ABCA1) were purchased from eBioscience (San Diego, CA, USA) and Novus Biologicals (Littleton, CO, USA), respectively. Anti-mouse 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) was purchased from Abcam (Cambridge, MA, USA), and all other chemicals and reagents used in this study were reagent grade.
Male C57BL/6NCrjBgi mice were purchased from the Charles River Laboratory of Animal Science (Orient Co., Seoul, Republic of Korea) at four weeks old and fed a normal diet for one week. Animals were housed in individual cages with free access to water and food in a temperature-controlled animal facility under a 12 h light-dark cycle at 22±2℃ and 55±5% humidity. Mice were fed either a high-fat diet (HFD) (Open Source diets #D12492; Research Diets Inc., New Brunswick, NJ) to induce obesity, or a regular diet (RD; Open Source diets #D12450B; Research Diets Inc.). The nutritional contents of the HFD were similar to those of the regular diet except for low carbohydrate content and a high level of fat.
At 26 weeks of age, mice exhibiting blood glucose levels >160 mg/dl were selected as non-insulin-dependent diabetes mellitus (NIDDM) animals and were divided into five groups of 20 animals per group. One group was administrated with PBS only and served as diabetic controls; four groups received daily 100 mg/kg of PAG, 2 mg/kg of Aloesin (ALS), 100 mg/kg of PAG containing 2% ALS (Aloe QDM), and Aloe QDM plus 500 mg/kg of Cr-enriched yeast containing 0.2% Cr (Aloe QDM complex) respectively, and the fifth group was administered pioglitazone (PGZ, 2.5 mg/kg), an anti-diabetic drug currently in clinical use. Mice were weighed and blood samples were collected weekly by tail bleeding into heparin-coated tubes after 4 h fasts.
At the end of the experimental period, mice were sacrificed and blood samples were taken from the inferior vena cava to determine plasma insulin and lipid levels. After collecting blood, the liver, thymus, pancreas, kidney, lung, heart, and spleen were removed, rinsed with physiological saline solution, and immediately stored at -70℃. White adipose tissues were immediately removed from periepididymal and perirenal fat for morphological examinations. Mice were treated in accordance with the guidelines issued by Sahmyook University for the care and use of laboratory animals.
Blood glucose concentrations were monitored after 4-hour fasts from venous blood from the tail vein using a glucometer (MediSence Optimum, Abbott Laboratories, Bedford, USA) at 7, 14, 24, 34, 44, and 54 days of age, equivalent to days of the supplementation period.
Blood was collected via cardiac puncture, allowed to clot for 30 minutes, and spun at 7,000 rpm for 10 minutes. Isolated serum was stored at -80℃. Plasma insulin levels were assayed using an enzyme-linked immunosorbent assay (ELISA) kit (Shibayagi's Insulin Assay Kit, Shibayagi Co., Gunma, Japan).
Adipose tissue was isolated from mice, fixed in 10% formalin, and embedded in paraffin. Four-micrometer thin sections were obtained, mounted on two glass-slides, and stained with hematoxylin-eosin. Sections were viewed with an Olympus microscope (Olympus, Tokyo, Japan) at ×400 magnification. Liver was isolated from mice, fixed in 10% formalin, and embedded in paraffin. Four-micrometer thin sections were obtained, mounted on two glass-slides, and stained with oil red O staining. Sections were viewed with an Olympus microscope (Olympus, Tokyo, Japan) at ×200 magnification.
Twenty mice were selected and used for this analysis. Liver and WAT tissues were immediately frozen in liquid nitrogen, and stored at -70℃ for the determination of gene transcripts. Total RNA was extracted from tissues using the RNeasy Mini kit (QIAGEN, Valencia, USA) in an RNase-free environment. RNA was then quantified by reading the absorbance at 260 nm. The reverse transcription of 1 µg RNA was carried out using M-MLV reverse transcriptase (Promega, USA), an oligo (dT) 16 primer, dNTP (0.5 µM), and 1 U RNase inhibitor. After incubation at 65℃ for 5 min and 37℃ for 60 min, M-MLV reverse transcriptase was inactivated by heating at 70℃ for 15 min. The polymerase chain reaction (PCR) was performed in 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, and 2.5 mM dNTPs with 5 units of Taq DNA polymerase and 10 pM of each primer set for Toll-like receptor 4 (TLR4), CCL5 (RANTES), peroxisome proliferator-activated receptor gamma (PPARγ), liver X receptor alpha (LXRα), and 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). The cDNA was amplified by 35 cycles of denaturing at 94℃ for 45 s, annealing at 62℃ for 45 s, and extension at 72℃ for 1 min. Final extension was performed at 72℃ for 5 min. The PCR products were then electrophoresed on a 1.5% agarose gels and stained with ethidium bromide. The primers selected were 5' TGA GAA GTC CCT GCT GAG GC 3' (forward) and 5' CTC CTC AGG TCC AAG TTG CC 3' (reverse) for TLR4, 5' ATC ATC CTC ACT GCA GCC GC 3' (forward) and 5' CAC ACT TGG CGG TTC CTT CG 3' (reverse) for CCL5, 5' GAG CCT GTG AGA CCA ACA GC 3' (forward) and 5' GAT TCC GAA GTT GGT GGG CC 3' (reverse) for PPARγ, 5' AGG GTT GGA GTC AGC AGA GC 3' (forward) and 5' GGA AGA ATC CCT TGC AGC CC 3' (reverse) for LXRα, 5' CAA GGC GGG AAA GCT CAT GG 3' (forward) and 5' GGA GGA GAT GAC GGC AAT GC 3' (reverse) for 11β-HSD1, and 5' CAA CTT TGG CAT TGT GGA AGG 3' (forward) and 5' ATG GAA ATT GTG AGG GAG ATG C 3' (reverse) for GAPDH, which was used as an internal control.
Frozen epididymal adipose tissue was homogenized in 3 volumes of ice lysis buffer (20 mM Trizma base, 50 mM NaCl, 250 mM sucrose, 50 mM NaF, 5 mM Na4P2O7·10H2O, 1% Triton-X100, 5 µg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 5 µg/ml aprotinin). Twenty micrograms of protein from the cell lysates was applied to 8~12% SDS-polyacrylamide gels and then transferred to nitrocellulose membranes. The membranes were blocked with 5% skim milk in TBST solution for 1 hr. They were then incubated with anti-i-NOS, anti-COX-2, anti-IL-1β, anti-IL-6, anti-IL-12, anti-TNF-α, anti-CX3CL1, anti-ABCA1, anti-11β-HSD1, anti-IL-4, and anti-IL-10 monoclonal antibodies for 2 hrs and washed 3 times with TBST. After incubation with alkaline phosphatase-labeled secondary antibody for 2 hrs, the bands were visualized using a Western Blot Kit with an alkaline phosphatase substrate (Vector, Burlingame, USA).
The obese mice supplemented with aloe formula had no difference in body weight compared to obese mice on a HFD (Fig. 1A). The effects of the aloe formula and PGZ supplementation on blood glucose levels have been shown in Fig. 1B. The HFD-fed mice exhibited a significant increase in blood glucose concentrations compared with RD fed mice. On the other hand, AADM complex-treated mice significantly decreased fasting blood glucose levels. Further, the plasma insulin levels of HFD-fed mice were significantly (3-fold) higher than those of regular diet-fed mice. And, treatment of HFDfed mice with aloe formula for 8 weeks significantly reduced plasma insulin levels (Fig. 1C).
Insulin resistance and diabetes can trigger hepatic steatosis, which has been associated with inflammation of the liver (25). To examine whether dietary aloe formula could suppress hepatic TG and inflammatory responses in the liver. Histological analysis showed that TG in the liver, which was numerous in the HFD group, completely disappeared after aloe formula supplementation, especially after Aloe QDM, supplementation (Fig. 2A). In agreement with this, levels of chemokines (CCL5), Toll-like receptor 4 (TLR4), and PPARγ/LXRα. Levels of CCL5 and TLR4 mRNA in the liver were significantly lower in obese mice supplemnted with dictary aloe formula than the HFD group (Fig. 3A). Also, the aloe formula decreased mRNA expression of PPARγ/LXRα (Fig. 4A).
These finding indicated that the dietary aloe formula attenuated obesity-induced hepatic steatosis and inflammation.
Histological analysis showed that the number of large adipocytes was lesser in the WAT of the diet-induced obesity (DIO) mice supplemented with aloe formula than in that of the HFD group, whereas there were more small adipocytes in the WAT of the of the DIO mice supplemented with aloe formula. In addition, fewer cells had infiltrated the WAT of the DIO mice receiving dietary aloe formula, especially Aloe QDM, than those in the HFD group (Fig. 2B).
The nuclear receptor, PPARγ, is endogenously activated by some polyunsaturated fatty acids and products of lipid metabolism. PPARγ activation has been shown to significantly attenuate adipocytes hypertrophy and inhibit WAT inflammation, whereas it increases the total WAT mass. We examined whether the aloe formula could affect mRNA expression of the nuclear receptor PPARγ/LXRα in WAT. PPARγ and LXR α mRNA expression was lower in WAT than in the PGZ group (Fig. 4A). We therefore examined the ability of PPARγ and LXRα ligands to modulate expression of ABCA1 in WAT. As shown in Fig. 4B, the dietary aloe formula-treated mice group exhibited a marked inhibition of ABCA1 protein expression in WAT.
It has been proven that an increase in 11β-HSD1 mRNA and activity is essential for the induction of adipogenesis by regulating the local level of glucocorticoids in WAT (26). The mRNA and protein expression of 11β-HSD1 was significantly decreased by aloe formula, but not in the PGZ group in WAT (Fig. 4C and D). This was noteworthy to that results of our data, and it was also confirmed that the aloe formula could be adopted as a PPAR antagonist and 11β-HSD1 inhibitor for the treatment of T2D.
To examine whether the dietary aloe formula modulated adipocytokine dysregulation,we measured adipocytokine gene and protein expression in WAT by RT-PCR and western blot. As shown in Fig. 3B and C, proinflammatory cytokines, IL-1β, IL-6, IL-12, and TNF-α, and chemokine (CX3CL1) protein were lower in the obese mice supplemented with aloe formula than in the HFD group. Cytokine-related enzymes, such as iNOS and COX-2 were also reduced by aloe formula, especially Aloe QDM. On the other hand, the anti-inflammatory cytokines, IL-4 and IL-10 protein in WAT was higher in the obese mice supplemented with aloe formula than in the HFD group (Fig. 5). These findings indicate that aloe formula, especially Aloe QDM reduces WAT inflammatory responses by preventing dysregulation of adipocytokine release.
This study demonstrated that aloe formula can reduce obesity-induced insulin resistance, and that the beneficial effect of aloe formula was associated with attenuation of inflammatory phenotypes in the WAT and liver, both of which have been shown to be important peripheral tissues for the insulin response.
Herbal prescriptions have been recognized as potentially valid by the scientific medical establishment, and their use has been increasing. Since traditional herbal prescriptions are generally prepared from a combination of crude drugs, on the basis of oriental prescriptions, and herbology, they may exert combined effects that differ from the sum of the effects of the individual constituents (27).
One of the most effective of the currently available medications for T2D is the TZD class of insulin-sensitizing drugs. The TZDs function in binding to the nuclear receptor PPARγ. In WAT, PPARγ activation promotes adipogenesis and the differentiation of new adipocytes. Despite increasing total adipose tissue mass, TZDs have been believed to improve systemic insulin sensitive cells and increase the production of adiponectin, a glucose-sensitizing peptide that exerts anti-inflammatory properties (14).
Dietary aloe formula has been demonstrated to affect inflammation by virtue of an immunosuppression. Recent studies have suggested that inflamed adipocytes in the obese trigger the development of obesity-related metabolic disorders such as insulin resistance and T2D, indicating that the reduction of tissue inflammation may be beneficial in obesity-related metabolic diseases. Our previous in vivo study demonstrated a potential effect of PAG, an aloe formula, on hypoglycemia and hypolipidemia (23). In particular, our results demonstrated that the administration of aloe formulas including PAG, ALS, Aloe QDM, and Aloe QDM complexes to these mice prevented the development of T2D-related symptoms. However, the prevention or a therapeutic effect on obesity-induced metabolic disorder have never been fully established. The most important finding in this study was that aloe formula reduced the impairment of obesity-induced inflammatory response. Other parameters such as proinflammatory cytokines, chemokines, and antiinflammatory cytokines also supported a reduction of inflammation through aloe formula supplementation in obese mice (Fig. 3 and 5). The administration of aloe formula to HFD-fed mice reduced blood glucose concentration to a normal level despite being continued throughout an 8-week treatment period (Fig. 1B) and further, it significantly improved insulin resistance (Fig. 1C). These results reveal that the aloe formula increased insulin sensitivity by decreasing blood glucose and insulin levels. These findings imply that aloe formulas, especially Aloe QDM oppose the development of an inflammation state and insulin resistance.
Transcriptional regulation by nuclear receptors such as PPARγ/LXRα and nuclear factor (NF)-κB has been demonstrated to be crucial for the inflammatory response (28). Further, PPARγ functions as a transcriptional regulator of cell differentiation and lipid metabolism. Despite increases in body fat mass, PPARγ agonists exert novel anti-diabetic effects. Our data demonstrated that an aloe formula decreased mRNA expression of PPARγ in liver and WAT of obese mice (Fig. 3A). In addition, hepatic TG was markedly reduced in the RD group by Aloe QDM supplementation (Fig. 2A). Recent studies have provided evidence that the nuclear receptors LXRα and LXRβ mediate the lipid induction of ABCA1 (29). Our data demonstrated that the aloe formula decreased mRNA expression of LXRα in liver and WAT of obese mice (Fig. 4A) and that the protein expression of ABCA1 was also decreased by Aloe QDM (Fig. 4B). We indicated that Aloe QDM suppressed the ability of PPARγ/LXRα to regulate ABCA1 expression in WAT leading to reduced total fat mass in obese mice.
It has been proven that an increase in 11β-HSD1 mRNA and activity is essential for the induction of adipogenesis by regulating the local level of glucocorticoids in WAT (26) and that the pharmacological inhibition of 11β-HSD1 could prevent adipocyte differentiation. Therefore, based on adipogenesis, we tested whether an aloe formula, affect the expression of 11β-HSD1, and 11β-HSD1 inhibition, might counteract the side effect of weight gain and show better anti-diabetic activity. Despite no change in body weight (Fig. 1A), Aloe QDM significantly reduced mRNA and protein expression of 11β-HSD1 in the both WAT and liver (Fig. 4C and D). In agreement with this, the histology of the adipose tissue from both PGZ-and Aloe QDM complex-treated groups exhibited a similar pattern and we observed that the relative numbers of adipocytes in the AADM complex-treated group was higher than in the HFD-fed mice and that it markedly reduced macrophage infiltration into WAT (Fig. 2B). Additionally, gene expression of obesity-induced inflammatory cytokines and chemokines were significantly suppressed in the Aloe QDM complex group, but not the PGZ group (Fig. 3), whereas the anti-inflammatory cytokines, Il-4 and Il-10, were significantly induced in the Aloe QDM complex group (Fig. 5).
In almost all data, Aloe QDM has been demonstrated to significantly improve insulin resistance and suppresse mRNA expression of proinflammatory cytokines in WAT and liver, suggesting that Cr supplementation with an aloe formula may support glucose uptake and led to regulation of insulin homeostasis in DIO mice.
In conclusion, aloe formulas suppresses obesity-induced inflammatory responses by reducing levels of the proinflammatory cytokines, PPARγ/LXRα, and 11β-HSD1, and by enhancing anti-inflammatory cytokines in WAT and liver, both of which are important peripheral tissues for insulin response. The beneficial effects of aloe formula with respect to obesity-induced insulin resistance and hepatic steatosis have been associated with its action on PPARγ/LXRα. In the current study, we demonstrated that Aloe QDM was a useful dietary phytochemical for improving not only obesity-induced inflammation but also obesity-related metabolic disorders.
Figures and Tables
Figure 1
Effects of aloe formulas on body weight change and obesity-induced glucose intolerance. C57BL/6 mice were fed a high-fat diet supplemented with an aloe formula. (A) Weekly changes in body weight of aloe formula-supplemented mice. (B) fasting glucose and (C) insulin levels in plasma. Data are means±SEM.values. ‡p<0.01 compared with RD-fed mice. *p<0.05, †p<0.01 compared with untreated HFD-fed mice.

Figure 2
Histological observations of liver and WAT. At the end of the experimental period, (A) fatty livers were isolated from representative mice from each group, sectioned, and then stained with hematoxylin and eosin. Photomicrographs are of tissues isolated from RD-fed mice (a), untreated HFD mice (b), PGZ (c), PAG (d), ALS (e), Aloe QDM (f), and Aloe QDM complex-treated HFD mice (g). Photomicrographs were taken at a magnification of ×200. (B) epididymal fat pads were isolated from mice, sectioned, and then stained with hematoxylin and eosin. Photomicrographs are of tissues isolated from RD-fed mice (a), untreated HFD mice (b), PGZ (c), and Aloe QDM complex-treated HFD mice (d). Arrows show infiltrated cells. Photomicrographs were taken at a magnification of ×400.

Figure 3
Effect of aloe formulas on obesity-induced inflammation. Liver and WAT were isolated from mice. (A) mRNA expression in the both liver and WAT were measured by RT-PCR. (B) protein expression of inflammatory cytokine-related enzymes and (C) proinflammatory cytokines and chemokines were measured by western blot. This was repeated in triplicate and similar results were obtained in all three.
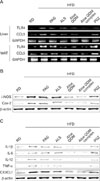
Figure 4
Effect of aloe formulas on adipogenesis. Liver and WAT were isolated from mice. (A) mRNA expression of PPARγ and LXRα in the both liver and WAT were measured by RT-PCR. (B) protein expression of ABCA1 was measured by western blot. (C) mRNA expression and (D) protein expression of 11β-HSD1 in both the liver and WAT were measured by RT-PCR and western blot, respectively. This was repeated in triplicate and similar results were obtained in all three.

ACKNOWLEDGEMENTS
This work was supported by Univera, Inc. as one of CAP projects and also by the Sahmyook University Research fund in 2010.
References
1. Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998. 41:1241–1248.

2. Grimble RF. Inflammatory status and insulin resistance. Curr Opin Clin Nutr Metab Care. 2002. 5:551–559.

3. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003. 112:1821–1830.

4. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003. 112:1796–1808.

5. Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997. 389:610–614.

6. Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004. 114:147–152.

7. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001. 409:307–312.

8. Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005. 436:356–362.

9. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010. 72:219–246.

10. Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004. 24:29–33.

11. Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001. 2:239–254.

12. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993. 259:87–91.

13. Wang P, Mariman E, Renes J, Keijer J. The secretory function of adipocytes in the physiology of white adipose tissue. J Cell Physiol. 2008. 216:3–13.

14. Josep BR, Amir G, Jennifer K, Raquel H. Peroxisome proliferator-activated receptors: the nutritionally controlled molecular networks that integrate inflammation, immunity and metabolism. Curr Nutr Food Sci. 2005. 1:179–187.

15. Capasso F, Borrelli F, Capasso R, Di Carlo G, Izzo A, Pinto L, Mascolo N, Castaldo S, Longo R. Aloe and its therapeutic use. Phytother Res. 1998. 12:S124–S127.

16. Heggers JP, Kucukcelebi A, Stabenau CJ, Ko F, Broemeling LD, Robson MC, Winters WD. Wound healing effects of Aloe gel and other topical antibacterial agents on rat skin. Phytother Res. 1995. 9:455–457.

18. Winters WD, Benavides R, Clouse WJ. Effects of aloe extracts on human normal and tumor cells in vitro. Econ Bot. 1981. 35:89–95.

19. Yongchaiyudha S, Rungpitarangsi V, Bunyapraphatsara N, Chokechaijaroenporn O. Antidiabetic activity of Aloe vera L. juice: I. Clinical trial in new cases of diabetes mellitus. Phytomedicine. 1996. 3:241–243.

20. Bunyapraphatsara N, Yongchaiyudha S, Rungpitarangsi V, Chokechaijaroenporn O. Antidiabetic activity of Aloe vera L. juice. II. Clinical trial in diabetes mellitus patients in combination with glibenclamide. Phytomedicine. 1996. 3:245–248.

21. Schwarz K, Mertz W. Chromium(III) and the glucose tolerance factor. Arch Biochem Biophys. 1959. 85:292–295.

23. Kim K, Kim H, Kwon J, Lee S, Kong H, Im SA, Lee YH, Lee YR, Oh ST, Jo TH, Park YI, Lee CK, Kim K. Hypoglycemic and hypolipidemic effects of processed Aloe vera gel in a mouse model of non-insulin-dependent diabetes mellitus. Phytomedicine. 2009. 16:856–863.

24. Kong H, Lee S, Shin S, Kwon J, Jo TH, Shin E, Shim KS, Park YI, Lee CK, Kim K. Down-regulation of adipogenesis and hyperglycemia in diet-induced obesity mouse model by Aloe QDM. Biomolecules Therapeutics. 2010. 18:336–342.

25. Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008. 7:496–507.

26. Kim J, Temple KA, Jones SA, Meredith KN, Basko JL, Brady MJ. Differential modulation of 3T3-L1 adipogenesis mediated by 11beta-hydroxysteroid dehydrogenase-1 levels. J Biol Chem. 2007. 282:11038–11046.

27. Kim JO, Kim KS, Lee GD, Kwon JH. Antihyperglycemic and antioxidative effects of new herbal formula in streptozotocin-induced diabetic rats. J Med Food. 2009. 12:728–735.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download