1. Craven LL. Experiences with aspirin (Acetylsalicylic acid) in the nonspecific prophylaxis of coronary thrombosis. Miss Valley Med J. 1953; 75:38–44. PMID:
13013156.
2. Steering Committee of the Physicians' Health Study Research Group. Final report on the aspirin component of the ongoing Physicians' Health Study. N Engl J Med. 1989; 321:129–135. PMID:
2664509.
3. Coller BS. Blockade of platelet GPIIb/IIIa receptors as an antithrombotic strategy. Circulation. 1995; 92:2373–2380. PMID:
7586333.

4. Leon MB, Baim DS, Popma JJ, Gordon PC, Cutlip DE, Ho KK, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med. 1998; 339:1665–1671. PMID:
9834303.
5. Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962; 194:927–929. PMID:
13871375.

6. Coller BS, Lang D, Scudder LE. Rapid and simple platelet function assay to assess glycoprotein IIb/IIIa receptor blockade. Circulation. 1997; 95:860–867. PMID:
9054743.

7. Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ten Berg JM, Hackeng CM. High on-aspirin platelet reactivity as measured with aggregation-based, cyclooxygenase-1 inhibition sensitive platelet function tests is associated with the occurrence of atherothrombotic events. J Thromb Haemost. 2010; 8:2140–2148. PMID:
20723029.

8. Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ruven HJ, Bal ET, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010; 303:754–762. PMID:
20179285.

9. Hansen ML, Sørensen R, Clausen MT, Fog-Petersen ML, Raunsø J, Gadsbøll N, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010; 170:1433–1441. PMID:
20837828.

10. Sibbing D, Steinhubl SR, Schulz S, Schömig A, Kastrati A. Platelet aggregation and its association with stent thrombosis and bleeding in clopidogrel-treated patients: initial evidence of a therapeutic window. J Am Coll Cardiol. 2010; 56:317–318. PMID:
20633826.
11. Ho PC, Triggs EJ, Bourne DW, Heazlewood VJ. The effects of age and sex on the disposition of acetylsalicylic acid and its metabolites. Br J Clin Pharmacol. 1985; 19:675–684. PMID:
4005105.

12. Dacey LJ, Munoz JJ, Johnson ER, Leavitt BJ, Maloney CT, Morton JR, et al. Effect of preoperative aspirin use on mortality in coronary artery bypass grafting patients. Ann Thorac Surg. 2000; 70:1986–1990. PMID:
11156107.

13. Mangano DT. Aspirin and mortality from coronary bypass surgery. N Engl J Med. 2002; 347:1309–1317. PMID:
12397188.

14. Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T, et al. Stopping vs. continuing aspirin before coronary artery surgery. N Engl J Med. 2016; 374:728–737. PMID:
26933848.

15. Cao L, Young N, Liu H, Silvestry S, Sun W, Zhao N, et al. Preoperative aspirin use and outcomes in cardiac surgery patients. Ann Surg. 2012; 255:399–404. PMID:
21997805.

16. Mazzeffi M, Galvagno S, Gammie JS, Tanaka K. Impact of aspirin use on morbidity and mortality in massively transfused cardiac surgery patients: a propensity score matched cohort study. J Anesth. 2016; 30:817–825. PMID:
27379496.

17. Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T, et al. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2017; 376:136–148. PMID:
27774838.

18. Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, et al. Stent Restenosis Study Investigators. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med. 1994; 331:496–501. PMID:
8041414.

19. Eikelboom JW, Hirsh J, Spencer FA, Baglin TP, Weitz JI. Antiplatelet drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141(2 Suppl):e89S–e119S. PMID:
22315278.
20. Hosokawa K, Ohnishi T, Sameshima H, Miura N, Ito T, Koide T, et al. Analysing responses to aspirin and clopidogrel by measuring platelet thrombus formation under arterial flow conditions. Thromb Haemost. 2013; 109:102–111. PMID:
23179055.

21. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016; 68:1082–1115. PMID:
27036918.
22. Ferraris VA, Boral LI, Cohen AJ, Smyth SS, White GC 2nd. Consensus review of the treatment of cardiovascular disease in people with hemophilia A and B. Cardiol Rev. 2015; 23:53–68. PMID:
25436468.

23. Kubota T, Chiba K, Ishizaki T. Genotyping of S-mephenytoin 4'-hydroxylation in an extended Japanese population. Clin Pharmacol Ther. 1996; 60:661–666. PMID:
8988068.

24. Nishio R, Shinke T, Otake H, Sawada T, Haraguchi Y, Shinohara M, et al. Effect of cytochrome P450 2C19 polymorphism on target lesion outcome after drug-eluting stent implantation in japanese patients receiving clopidogrel. Circ J. 2012; 76:2348–2355. PMID:
22785462.

25. Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010; 50:126–142. PMID:
19948947.

26. Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009; 373:723–731. PMID:
19249633.

27. Goodnough LT, Smith PK, Levy JH, Poston RS, Short MA, Weerakkody GJ, et al. Transfusion outcomes in patients undergoing coronary artery bypass grafting treated with prasugrel or clopidogrel: TRITON-TIMI 38 retrospective data analysis. J Thorac Cardiovasc Surg. 2013; 145:1077–1082. PMID:
22995726.

28. Saito S, Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS study. Circ J. 2014; 78:1684–1692. PMID:
24759796.
29. Nishikawa M, Isshiki T, Kimura T, Ogawa H, Yokoi H, Miyazaki S, et al. Risk of bleeding and repeated bleeding events in prasugrel-treated patients: a review of data from the Japanese PRASFIT studies. Cardiovasc Interv Ther. 2017; 32:93–105. PMID:
28097639.

30. Teng R, Oliver S, Hayes MA, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010; 38:1514–1521. PMID:
20551239.

31. Becker RC, Bassand JP, Budaj A, Wojdyla DM, James SK, Cornel JH, et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2011; 32:2933–2944. PMID:
22090660.

32. Hansson EC, Jidéus L, Åberg B, Bjursten H, Dreifaldt M, Holmgren A, et al. Coronary artery bypass grafting-related bleeding complications in patients treated with ticagrelor or clopidogrel: a nationwide study. Eur Heart J. 2016; 37:189–197. PMID:
26330426.

33. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011; 123:2736–2747. PMID:
21670242.
34. Norgard NB. Cangrelor: a novel P2Y12 receptor antagonist. Expert Opin Investig Drugs. 2009; 18:1219–1230.
35. Akers WS, Oh JJ, Oestreich JH, Ferraris S, Wethington M, Steinhubl SR. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: a direct, parenteral P2Y12 receptor antagonist. J Clin Pharmacol. 2010; 50:27–35. PMID:
19779037.

37. Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013; 368:1303–1313. PMID:
23473369.

38. Angiolillo DJ, Firstenberg MS, Price MJ, Tummala PE, Hutyra M, Welsby IJ, et al. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trial. JAMA. 2012; 307:265–274. PMID:
22253393.

39. Kirchmaier CM, Pillitteri D. Diagnosis and management of inherited platelet disorders. Transfus Med Hemother. 2010; 37:237–246. PMID:
21113246.

40. Flechtenmacher N, Kämmerer F, Dittmer R, Budde U, Michels P, Röther J, et al. Clopidogrel resistance in neurovascular stenting: correlations between light transmission aggregometry, verifynow, and the multiplate. AJNR Am J Neuroradiol. 2015; 36:1953–1958. PMID:
26272977.

41. Jakubowski JA, Payne CD, Li YG, Brandt JT, Small DS, Farid NA, et al. The use of the VerifyNow P2Y
12 point-of-care device to monitor platelet function across a range of P2Y
12 inhibition levels following prasugrel and clopidogrel administration. Thromb Haemost. 2008; 99:409–415. PMID:
18278193.
42. Nishi T, Ariyoshi N, Nakayama T, Fujimoto Y, Sugimoto K, Takahara M, et al. Increased platelet inhibition after switching from maintenance clopidogrel to prasugrel in Japanese patients with stable coronary artery disease. Circ J. 2015; 79:2439–2444. PMID:
26310876.

43. Benzon HT, McCarthy RJ, Benzon HA, Kendall MC, Robak S, Lindholm PF, et al. Determination of residual antiplatelet activity of clopidogrel before neuraxial injections. Br J Anaesth. 2011; 107:966–971. PMID:
21968250.

44. Kakouros N, Kickler TS, Laws KM, Rade JJ. Hematocrit alters VerifyNow P2Y
12 assay results independently of intrinsic platelet reactivity and clopidogrel responsiveness. J Thromb Haemost. 2013; 11:1814–1822. PMID:
24118870.
45. Sibbing D, Schulz S, Braun S, Morath T, Stegherr J, Mehilli J, et al. Antiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placement. J Thromb Haemost. 2010; 8:250–256. PMID:
19943882.

46. Weber CF, Görlinger K, Meininger D, Herrmann E, Bingold T, Moritz A, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012; 117:531–547. PMID:
22914710.
47. Mazzeffi M, Lund L, Wallace K, Herrera AV, Tanaka K, Odonkor P, et al. Effect of cardiopulmonary bypass on platelet mitochondrial respiration and correlation with aggregation and bleeding: a pilot study. Perfusion. 2016; 31:508–515. PMID:
26916901.

48. Hobson AR, Petley GW, Dawkins KD, Curzen N. A novel fifteen minute test for assessment of individual time-dependent clotting responses to aspirin and clopidogrel using modified thrombelastography. Platelets. 2007; 18:497–505. PMID:
17957565.

49. Sambu N, Hobson A, Curzen N. “Short” thrombelastography as a test of platelet reactivity in response to antiplatelet therapy: validation and reproducibility. Platelets. 2011; 22:210–216. PMID:
21231856.

50. Collyer TC, Gray DJ, Sandhu R, Berridge J, Lyons G. Assessment of platelet inhibition secondary to clopidogrel and aspirin therapy in preoperative acute surgical patients measured by Thrombelastography Platelet Mapping. Br J Anaesth. 2009; 102:492–498. PMID:
19286767.

51. Mahla E, Suarez TA, Bliden KP, Rehak P, Metzler H, Sequeira AJ, et al. Platelet function measurement-based strategy to reduce bleeding and waiting time in clopidogrel-treated patients undergoing coronary artery bypass graft surgery: the timing based on platelet function strategy to reduce clopidogrel-associated bleeding related to CABG (TARGET-CABG) study. Circ Cardiovasc Interv. 2012; 5:261–269. PMID:
22396581.

52. Xiao Z, Théroux P. Platelet activation with unfractionated heparin at therapeutic concentrations and comparisons with a low-molecularweight heparin and with a direct thrombin inhibitor. Circulation. 1998; 97:251–256. PMID:
9462526.

53. Mochizuki T, Olson PJ, Szlam F, Ramsay JG, Levy JH. Protamine reversal of heparin affects platelet aggregation and activated clotting time after cardiopulmonary bypass. Anesth Analg. 1998; 87:781–785. PMID:
9768770.

54. Michelson AD, MacGregor H, Barnard MR, Kestin AS, Rohrer MJ, Valeri CR. Reversible inhibition of human platelet activation by hypothermia in vivo and in vitro. Thromb Haemost. 1994; 71:633–640. PMID:
7522354.

55. Hoffmeister KM, Felbinger TW, Falet H, Denis CV, Bergmeier W, Mayadas TN, et al. The clearance mechanism of chilled blood platelets. Cell. 2003; 112:87–97. PMID:
12526796.

56. Karkouti K, McCluskey SA, Syed S, Pazaratz C, Poonawala H, Crowther MA. The influence of perioperative coagulation status on postoperative blood loss in complex cardiac surgery: a prospective observational study. Anesth Analg. 2010; 110:1533–1540. PMID:
20435945.
57. Velik-Salchner C, Maier S, Innerhofer P, Kolbitsch C, Streif W, Mittermayr M, et al. An assessment of cardiopulmonary bypass-induced changes in platelet function using whole blood and classical light transmission aggregometry: the results of a pilot study. Anesth Analg. 2009; 108:1747–1754. PMID:
19448196.

58. Van Poucke S, Stevens K, Wetzels R, Kicken C, Verhezen P, Theunissen M, et al. Early platelet recovery following cardiac surgery with cardiopulmonary bypass. Platelets. 2016; 27:751–757. PMID:
27164510.

59. Rahe-Meyer N, Winterhalter M, Boden A, Froemke C, Piepenbrock S, Calatzis A, et al. Platelet concentrates transfusion in cardiac surgery and platelet function assessment by multiple electrode aggregometry. Acta Anaesthesiol Scand. 2009; 53:168–175. PMID:
19175576.

60. Weitzel NS, Weitzel LB, Epperson LE, Karimpour-Ford A, Tran ZV, Seres T. Platelet mapping as part of modified thromboelastography (TEG®) in patients undergoing cardiac surgery and cardiopulmonary bypass. Anaesthesia. 2012; 67:1158–1165. PMID:
22809250.

61. Orlov D, McCluskey SA, Callum J, Rao V, Moreno J, Karkouti K. Utilization and effectiveness of desmopressin acetate after cardiacsurgery supplemented with point-of-care hemostatic testing: a propensity-score-matched analysis. J Cardiothorac Vasc Anesth. 2017; 31:883–895. PMID:
28169116.
62. Karkouti K, Callum J, Wijeysundera DN, Rao V, Crowther M, Grocott HP, et al. Point-of-care hemostatic testing in cardiac surgery: a stepped-wedge clustered randomized controlled trial. Circulation. 2016; 134:1152–1162. PMID:
27654344.
63. Zafar MU, Santos-Gallego C, Vorchheimer DA, Viles-Gonzalez JF, Elmariah S, Giannarelli C, et al. Platelet function normalization after a prasugrel loading-dose: time-dependent effect of platelet supplementation. J Thromb Haemost. 2013; 11:100–106. PMID:
23137352.

64. Hansson EC, Shams Hakimi C, Åström-Olsson K, Hesse C, Wallén H, Dellborg M, et al. Effects of ex vivo platelet supplementation on platelet aggregability in blood samples from patients treated with acetylsalicylic acid, clopidogrel, or ticagrelor. Br J Anaesth. 2014; 112:570–575. PMID:
24148324.

65. Vilahur G, Choi BG, Zafar MU, Viles-Gonzalez JF, Vorchheimer DA, Fuster V, et al. Normalization of platelet reactivity in clopidogreltreated subjects. J Thromb Haemost. 2007; 5:82–90. PMID:
17239165.

66. Akay OM, Gündüz E, Başyiğit H, Gulbas Z. Platelet function testing during 5-day storage of single and random donor plateletpheresis. Transfus Apher Sci. 2007; 36:285–289. PMID:
17602871.

67. Prüller F, Drexler C, Archan S, Macher S, Raggam RB, Mahla E. Low platelet reactivity is recovered by transfusion of stored platelets: a healthy volunteer in vivo study. J Thromb Haemost. 2011; 9:1670–1673. PMID:
21649849.
68. Dalén M, Ivert T, Lindvall G, van der Linden J. Ticagrelor-associated bleeding in a patient undergoing surgery for acute type A aortic dissection. J Cardiothorac Vasc Anesth. 2013; 27:e55–e57. PMID:
23921293.

69. Hedner U. NovoSeven as a universal haemostatic agent. Blood Coagul Fibrinolysis. 2000; 11(Suppl 1):S107–S111. PMID:
10850574.

70. Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010; 363:1791–1800. PMID:
21047223.

71. Skolnick BE, Shenouda M, Khutoryansky NM, Pusateri AE, Gabriel D, Carr ME. Reversal of clopidogrel-induced bleeding with rFVIIa in healthy subjects: a randomized, placebo-controlled, double-blind, exploratory study. Anesth Analg. 2011; 113:703–710. PMID:
21890888.
72. Gill R, Herbertson M, Vuylsteke A, Olsen PS, von Heymann C, Mythen M, et al. Safety and efficacy of recombinant activated factor VII: a randomized placebo-controlled trial in the setting of bleeding after cardiac surgery. Circulation. 2009; 120:21–27. PMID:
19546387.
73. Branchford BR, Di Paola J. Making a diagnosis of VWD. Hematology Am Soc Hematol Educ Program. 2012; 2012:161–167. PMID:
23233576.

74. Mannucci PM. Desmopressin (DDAVP) in the treatment of bleeding disorders: the first 20 years. Blood. 1997; 90:2515–2521. PMID:
9326215.

75. Crescenzi G, Landoni G, Biondi-Zoccai G, Pappalardo F, Nuzzi M, Bignami E, et al. Desmopressin reduces transfusion needs after surgery: a meta-analysis of randomized clinical trials. Anesthesiology. 2008; 109:1063–1076. PMID:
19034103.
76. Swieringa F, Lancé MD, Fuchs B, Feijge MA, Solecka BA, Verheijen LP, et al. Desmopressin treatment improves platelet function under flow in patients with postoperative bleeding. J Thromb Haemost. 2015; 13:1503–1513. PMID:
25988848.

77. Ferraris VA, Saha SP, Oestreich JH, Song HK, Rosengart T, Reece TB, et al. 2012 update to the Society of Thoracic Surgeons guideline on use of antiplatelet drugs in patients having cardiac and noncardiac operations. Ann Thorac Surg. 2012; 94:1761–1781. PMID:
23098967.

78. Trenk D, Stone GW, Gawaz M, Kastrati A, Angiolillo DJ, Müller U, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J Am Coll Cardiol. 2012; 59:2159–2164. PMID:
22520250.
79. Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008; 358:2319–2331. PMID:
18480196.

80. Martin K, Wiesner G, Breuer T, Lange R, Tassani P. The risks of aprotinin and tranexamic acid in cardiac surgery: a one-year follow-up of 1188 consecutive patients. Anesth Analg. 2008; 107:1783–1790. PMID:
19020118.

81. Witzenbichler B, Maehara A, Weisz G, Neumann FJ, Rinaldi MJ, Metzger DC, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014; 129:463–470. PMID:
24281330.
82. Feres F, Costa RA, Abizaid A, Leon MB, Marin-Neto JA, Botelho RV, et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013; 310:2510–2522. PMID:
24177257.
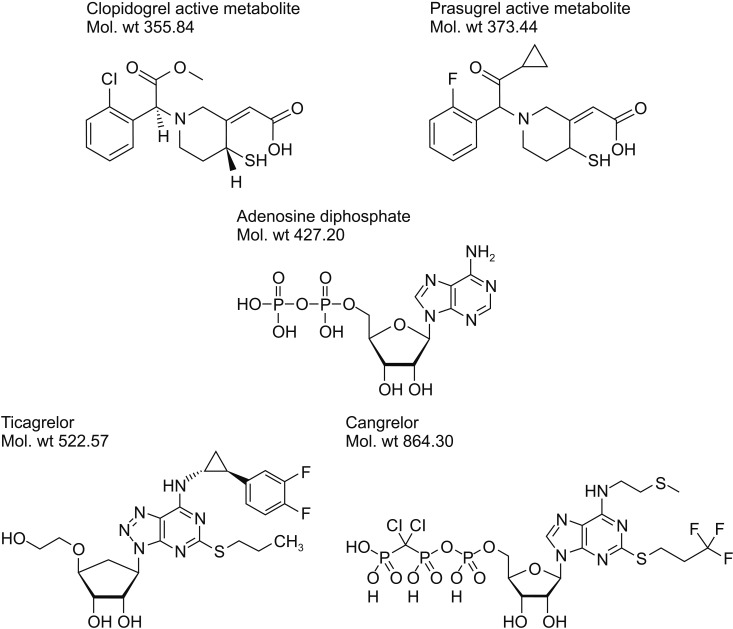
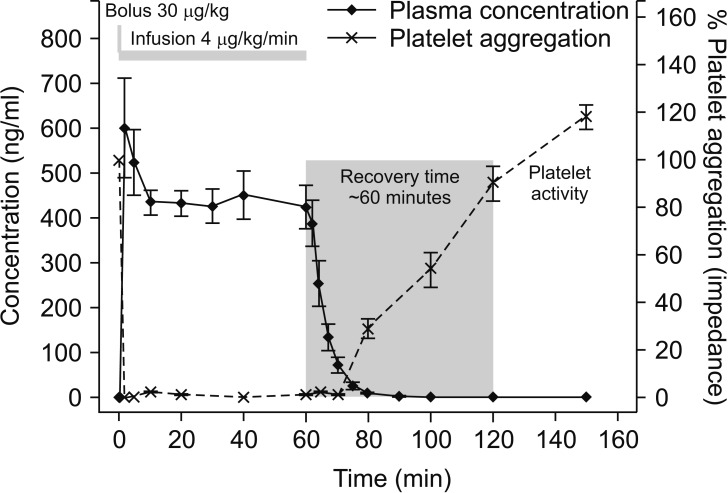
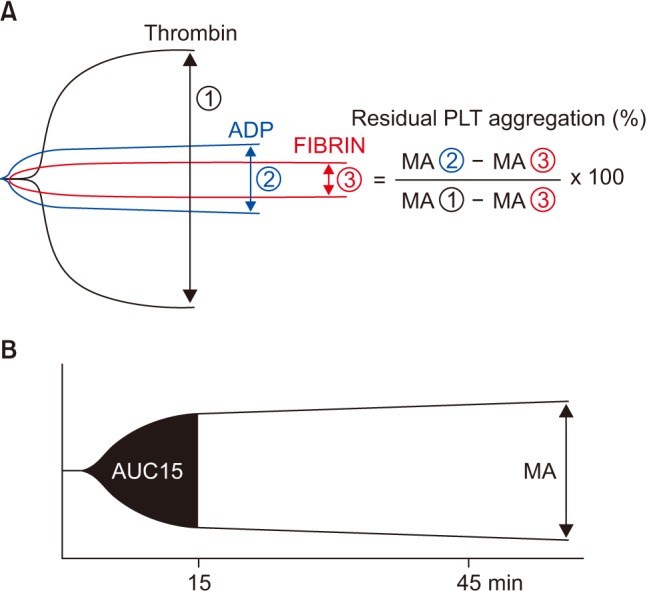




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download