Abstract
Background
Despite a recent breakthrough in human islet transplantation for treating diabetes mellitus, the limited availability of insulin-producing tissue is still a major obstacle. This has led to a search for alternative sources of transplantable insulin-producing cells including pancreatic duct cells. We aimed to establish in vitro culture of pancreatic duct cells from a partial pancreas tissue in human, which could be harnessed to differentiate into pancreatic β cells.
Methods
We isolated pancreatic duct cells from small pieces of pancreas tissue (1~3 g) derived from non-diabetic humans (n = 8) undergoing pancreatic surgery due to cancer. Pancreas tissue was finely minced after injection of collagenase P into the parenchyma. The mince was incubated in a shaking water bath at 37℃ for 25 min and passed through a 150 µm mesh. The released cells were recovered, washed, and plated in a dish containing CMRL culture medium with serum.
Results
Isolated pancreatic cells grew in monolayer and became confluent in 1~2 wks showing typical epithelial cobblestone morphology. Immunochemistry demonstrated that ~90% of the cultured cells were cytokeratin7-positive duct cells. To induce β cell differentiation, the cells were incubated in DMEM/F12 culture medium without serum. In addition, treatment with Matrigel overlay, exendin-4, cholera toxin or forskolin was done. Though β cell differentiation was found by immunostaining and RT-PCR, the differentiation efficiency was very low. Over-expression of neurogenin-3 by recombinant adenovirus did not increase β cell differentiation of the cultured duct cells significantly.
Figures and Tables
Fig. 3
Cytokeratin 7 (CK7) immunostaining. A, human pancreas (×100); B, human pancreas (×200); C, Isolated pancreatic cells; D, Cultured pancreatic cells 4 days after isolation.
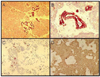
Fig. 4
Double immunostaining of CK7 and insulin in pancreatic duct cells cultured in differentiation medium for 1 week.
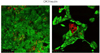
Fig. 5
Matrigel overlay on pancreatic duct cells cultured in differentiation medium for 4 week. A, B, morphology under inverted microscope; C, CK7 staining; D, insulin staining.
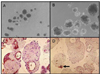
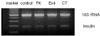
Fig. 6
RT-PCR of insulin mRNA from pancreatic duct cells 3 days after treatment of forskolin (FK), exendin-4 (Ex4) or cholera toxin (CT).
References
1. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000. 343:230–238.
2. Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004. 350:694–705.
3. Bouwens L. Cytokeratins and cell differentiation in the pancreas. Journal of Pathology. 1998. 184:234–239.
4. Kim SK, MacDonald RJ. Signaling and transcriptional control of pancreatic organogenesis. Curr Opin Genet Dev. 2002. 12:540–547.
5. Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of the dult aexocrine and endocrine pancreas: A possible recapitulation of embryonic development. Diabetes. 1993. 42:1715–1720.
6. Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song K-H, Sharma A, O'Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000. 97:7999–8004.
7. Heremans Y, Van De Casteele M, in't Veld P, Gradwohl G, Serup P, Madsen O, Pipeleers D, Heimberg H. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J Cell Biol. 2002. 159:303–312.
8. Gao R, Ustinov J, Pulkkinen MA, Lundin K, Korsgren O, Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2003. 52:2007–2015.
9. Zhao M, Amiel SA, Christie MR, Rela M, Heaton N, Huang GC. Insulin-producing cells derived fromhuman pancreatic non-endocrine cell cultures reverse streptozotocin-induced hyperglycaemia in mice. Diabetologia. 2005. 48:2051–2061.
10. Gao R, Ustinov J, Korsgren O, Otonkoski T. In vitro neogenesis of human islets reflects the plasticity of differentiated human pancreatic cells. Diabetologia. 2005. 48:2296–2304.
11. Hao E, Tyrberg B, Itkin-Ansari P, Lakey JR, Geron I, Monosov EZ, Barcova M, Mercola M, Levine F. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med. 2006. 12:310–316.
12. Kolar C, Caffrey T, Hollingsworth M, Scheetz M, Sutherlin M, Weide L, Lawson T. Duct epithelial cells cultured from human pancreas processed for transplantation retain differentiated ductal characteristics. Pancreas. 1997. 15:265–271.
13. Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999. 400:877–881.
14. Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000. 127:3533–3542.
16. Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, Bonner-Weir S. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999. 48:507–513.
17. Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004. 429:41–46.
18. Zhou J, Pineyro MA, Wang X, Doyle ME, Egan JM. Exendin-4 differentiation of a human pancreatic duct cell line into endocrine cells: involvement of PDX-1 and HNF3beta transcription factors. J Cell Physiol. 2002. 192:304–314.
19. Wang R, Li J, Rosenberg L. Factors mediating the transdifferentiation of islets of Langerhans to duct epithelial-like structures. J Endocrinol. 2001. 171:309–318.
20. Harb G, Heremans Y, Heimberg H, Korbutt GS. Ectopic expression of neurogenin 3 in neonatal pig pancreatic precursor cells induces (trans) differentiation to functional alpha cells. Diabetologia. 2006. 49:1855–1863.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download