Abstract
Background
Chronic hyperglycemia has deleterious effects on pancreatic β-cell function and turnover. Recent studies support the view that cyclin-dependent kinase 5 (CDK5) plays a role in β-cell failure under hyperglycemic conditions. However, little is known about how CDK5 impair β-cell function. Myricetin, a natural flavonoid, has therapeutic potential for the treatment of type 2 diabetes mellitus. In this study, we examined the effect of myricetin on high glucose (HG)-induced β-cell apoptosis and explored the relationship between myricetin and CDK5.
Methods
To address this question, we subjected INS-1 cells and isolated rat islets to HG conditions (30 mM) in the presence or absence of myricetin. Docking studies were conducted to validate the interaction between myricetin and CDK5. Gene expression and protein levels of endoplasmic reticulum (ER) stress markers were measured by real-time reverse transcription polymerase chain reaction and Western blot analysis.
Results
Activation of CDK5 in response to HG coupled with the induction of ER stress via the down regulation of sarcoendoplasmic reticulum calcium ATPase 2b (SERCA2b) gene expression and reduced the nuclear accumulation of pancreatic duodenal homeobox 1 (PDX1) leads to β-cell apoptosis. Docking study predicts that myricetin inhibit CDK5 activation by direct binding in the ATP-binding pocket. Myricetin counteracted the decrease in the levels of PDX1 and SERCA2b by HG. Moreover, myricetin attenuated HG-induced apoptosis in INS-1 cells and rat islets and reduce the mitochondrial dysfunction by decreasing reactive oxygen species production and mitochondrial membrane potential (Δψm) loss.
Progressive β-cell failure is one of the key pathogenic events in the development and progression of type 2 diabetes mellitus (T2DM) [1]. Although the molecular signals that trigger progressive deterioration of β-cell function are poorly understood, it is clear that chronic exposure to hyperglycemia can cause β-cell failure [2], and endoplasmic reticulum (ER) stress is an important contributor to hyperglycemia-induced β-cell failure [3]. ER stress can cause β-cell apoptosis through the induction of mitochondrial dysfunction and subsequent activation of the mitochondria-dependent apoptotic pathway [45].
Cyclin-dependent kinase 5 (CDK5) is a serine/threonine kinase with predominantly neuronal expression, which forms active complexes with its activators, such as p35 [6]. Previous studies showed that CDK5 activation promotes mitochondrial dysfunction and apoptosis in neurons [78]. Recently, accumulating evidence suggests that CDK5 and p35 are also present in pancreatic β-cells and play an important role in high glucose (HG)-induced β-cell failure [9101112].
Myricetin (3,5,7,3′,4′,5′-hexahydroxyflavone) is a natural flavonoid abundant in fruits, vegetables, tea, and berries and exhibits a wide range of effects, including anti-inflammatory, anti-oxidant, and anti-carcinogenic activities [13]. Recent studies show that myricetin ameliorates insulin resistance in animal models of T2DM [141516] and protects β-cells against cytokine-induced apoptosis [17]. These results suggest that myricetin might be a potential therapeutic agent for the treatment of T2DM. However, the effect of myricetin on β-cells under HG conditions has not been investigated yet. In the present study, we examined whether myricetin exhibits protective effects against HG-induced apoptosis in β-cells and explored the relationship between myricetin and CDK5.
Myricetin and roscovitine were purchased from Sigma-Aldrich (St Louis, MO, USA). Antibodies against pancreatic duodenal homeobox 1 (PDX1), caspase-3, B-cell lymphoma 2 (Bcl-2), Bax, phosphorylated c-Jun N-terminal kinase (P-JNK), phosphorylated eukaryotic initiation factor 2α (P-eIF2α), phosphorylated protein kinase R-like endoplasmic reticulum kinase (P-PERK), were from Cell Signaling Technology (Danvers, MA, USA). The antibody against cytochrome c was purchased from BD Biosciences (San Jose, CA, USA), and the antibody against β-actin was from Abcam (Cambridge, UK). Antibodies against phosphorylated CDK5 (tyrosine 15 [Tyr15]), CDK5, p35, activating transcription factor 4 (ATF4), CCAAT-enhancer-binding protein homologous protein (CHOP), glucose regulated protein 78 (Grp78) and sarcoendoplasmic reticulum calcium ATPase 2 (SERCA2) were from Santa Cruz (Dallas, TX, USA).
The INS-1 rat insulinoma cell line was cultured at 5% CO2/95% air at 37℃ in RPMI-1640 (Gibco BRL, Grand Island, NY, USA) containing 11.2 mM glucose and 2 mM L-glutamine. The medium was supplemented with 10% fetal bovine serum, 1 mM pyruvate, 10 mM HEPES, 50 µM 2-mercaptoethanol, 100 units/mL penicillin, and 100 µg/mL streptomycin. Pancreatic rat islets were isolated from male Sprague-Dawley rats (250 to 300 g) by collagenase digestion technique as previously described [18]. All animal procedures were approved by the Kyungpook National University Animal Care and Use Committee (KNU-2012-0001).
Total RNA was obtained from INS-1 cells using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed using the First Strand cDNA synthesis kit (Fermentas, Vilnius, Lithuania) according to manufacturer's instructions. Real-time polymerase chain reaction was performed using a Light Cycler instrument (Roche, Mannheim, Germany) with the following primers: 5′-ACC CAA GTC CCG TCG TGA AGT-3′ (forward) and 5′-CCA GTT GGT AGA GGG AGC AGA TG-3′ (reverse) for insulin; 5′-GGC TTA ACC TAA ACG CCA CA-3′ (forward) and 5′-GGG ACC GTC CAA GTT TGT AA-3′ (reverse) for PDX1; 5′-GTG GAA CCT TTG CCA CTC AT-3′ (forward) and 5′-TGT GCT GTA GAC CCA GAC CA-3′ (reverse) for SERCA2b; and 5′-TAC TGC CCT GGC TCC TAG CA-3′ (forward) and 5′-TGG ACA GTG AGG CCA GGA TAG-3′ (reverse) for β-actin. The expression level of β-actin was used as internal control.
Apoptosis was assessed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using an in situ cell death detection kit (Roche, Basel, Switzerland). INS-1 cells and primary islets were incubated with 30 mM glucose for 24 or 48 hours, in the presence or absence of myricetin. After incubation, cells were washed with 1X phosphate-buffered saline (PBS) for three times, fixed with 2% paraformaldehyde for 15 minutes, and then permeabilized with 0.2% Triton X-100 for 10 minutes at room temperature. After permeabilization, cells were washed again with PBS and processed further, according to the manufacturer's instructions. Images were captured using a fluorescence microscope. Islet cells with TUNEL-positive nuclei were considered apoptotic, and the percentage of TUNEL-positive cells relative to total cell number was determined. Cell viability was measured using the Cell Counting Kit-8 (Dojindo Laboratories, Kamimashiki, Japan) according to the manufacturer's instructions.
Δψm was assessed using 3,3′-dihexyloxacarbocyanine iodide (DiOC6; Sigma-Aldrich). Briefly, cells were washed once with PBS and then labeled with 10 nM DiOC6 for 5 minutes at 37℃. The cells were washed once and the cell fluorescence was analyzed using a flow cytometer (BD Biosciences). Intracellular reactive oxygen species (ROS) generation was measured using 2′, 7′-dichlorodihydrofluorescein diacetate (DCF-DA, Molecular Probes; Invitrogen). Cells were incubated in the dark for 15 minutes with 10 µM DCF-DA at 37℃ and then visualized under a fluorescence microscope. The mean fluorescence intensity was used to quantify cellular ROS.
Cell lysates were prepared using a lysis buffer (20 mM Tris-HCL pH7.4, 10 mM Na4P2OH, 100 mM NaF, 2 mM Na3VO4, 5 mM ethylenediaminetetraacetic acid [EDTA] pH 8.0, 0.1 mM phenylmethylsulfonyl fluoride [PMSF], 1% NP-40) containing protease and phosphatase inhibitors. Proteins were resolved by 4% to 15% SDS-polyacrylamide gradient gel and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). After blocking, the membranes were incubated with primary antibodies, washed, and incubated with a horseradish peroxidase-conjugated secondary antibody. Immunoreactive proteins were detected using ECL reagents (ECL Plus; Amersham, GE Healthcare Life Sciences, Little Chalfont, UK).
INS-1 cells were grown on glass coverslips for 2 days in culture medium. After the appropriate treatment, cells were fixed in 2% paraformaldehyde for 15 minutes and then permeabilized with 0.2% Triton X-100 for 15 minutes at room temperature. Cells were incubated with a primary antibody against PDX1 overnight and then with the secondary antibody Alexa-Fluor488 (Invitrogen) for 1 hour. The cells were visualized using a confocal microscope (Fluoview FV1000; Olympus, Tokyo, Japan).
For the binding model prediction of myricetin and the CDK5 kinase domain, myricetin was built using the Maestro build panel and the energy minimization method of the MacroModel in the Schrödinger software package. The crystal structure of CDK5 bound with roscovitine was used for the docking simulation (pdb code: 1 UNL). The protein structure was minimized using the Protein Preparation Wizard (Schrödinger, New York, NY, USA) by applying an OPLS-2005 force field. The prepared protein and the ligand were employed to build energy grids using the default value of protein atom scaling (1.0 Å) within a cubic box, defined as the centroid of the roscovitine-binding pocket of CDK5. After grid generation, the ligand was docked with the protein by using Glide module (Glide version 6.9, 2015; Schrödinger) in extra precision mode (XP). The best-docked poses were selected as the lowest Glide score.
INS-1 cells were pre-incubated with myricetin (20 µM) for 1 hour (5% CO2, 37℃) in RPMI medium and washed twice in Krebs-Ringer bicarbonate buffer (114 mmol/L NaCl, 4.4 mmol/L KCl, 1.28 mmol/L CaCl2, 1 mmol/L MgSO4, 29.5 mmol/L NaHCO3, 10 mmol/L HEPES, 2.8 mmol/L glucose, and 0.1% bovine serum albumin, pH 7.4). After that cells were incubated for 1 hour in krebs ringer bicarbonate buffer with the basal (2.8 mmol/L) or the stimulatory (16.6 mmol/L) glucose with or without myricetin. The supernatant was carefully collected and subjected to rat insulin radioimmunoassay (Linco Research, St. Charles, MO, USA).
We first examined the effect of myricetin, whose chemical structure is reported in Fig. 1A, on cell viability in INS-1 cells. As shown in Fig. 1B, treatment with myricetin at concentrations between 0.1 and 100 µM, for 24 hours, did not significantly affect cell viability. In a previous study, cytokine-induced apoptosis in β-cells was strongly attenuated by myricetin at a concentration of 20 µM [17]. Accordingly, we decided to use a myricetin concentration of 20 µM in all subsequent experiments. We next evaluated whether myricetin protects INS-1 cells from HG-induced apoptosis. As determined by TUNEL assays, myricetin treatment dose-dependently inhibited apoptosis in INS-1 cells incubated with 30 mM glucose for 24 hours (Fig. 1C). Furthermore, myricetin significantly attenuated apoptosis in isolated rat islets incubated with 30 mM glucose for 48 hours (Fig. 1D).
Increased ROS accumulation and increased mitochondrial membrane potential (Δψm) loss are characteristic of mitochondrial dysfunction [19]. Therefore, we next evaluated the effects of myricetin on mitochondrial function in INS-1 cells exposed to HG by measuring ROS generation and Δψm. As shown in Fig. 2A, myricetin treatment reduced the generation of ROS in INS-1 cells under HG conditions. In addition, the enhanced effect of HG on mitochondrial potential (Δψm) loss was significantly counteracted by myricetin (Fig. 2B). Myricetin also suppressed the increase in the expression of cytosolic cytochrome c and cleaved caspase-3 induced by HG (Fig. 2C). Exposure to HG increased Bax expression and decreased Bcl-2 expression and these changes were reversed by myricetin treatment (Fig. 2D).
Previous studies showed that CDK5 activation contributes to mitochondrial dysfunction and apoptosis in neuronal cells [78] and pancreatic β-cells [9101112]. As expected, we observed that treatment with roscovitine, a potent CDK5 inhibitor, attenuated the increase of both apoptosis and expression of cleaved caspase-3 induced by HG (Fig. 3A and B). Based on these results, we hypothesized that myricetin protects β-cells from HG-induced apoptosis, possibly through inactivation of CDK5. To test our hypothesis, we evaluated the effect of myricetin on CDK5 in cells exposed to HG. As shown in Fig. 3C, the phosphorylation of CDK5 on Tyr15 was increased from 2 to 24 hours of culture in HG conditions. Myricetin treatment significantly inhibited the increase of phosphorylated CDK5. Notably, total CDK5 was not affected by HG and myricetin. Moreover, the expression of p35 was also increased in HG-exposed cells and myricetin prevented this increase (Fig. 3C). Because an in vitro kinase assay revealed the inhibitory effects of myricetin on the activity of CDK5 [20], we conducted docking studies to validate the interaction between myricetin and CDK5. The proposed binding model of myricetin and CDK5 is reported in Fig. 3D. In this predicted model, myricetin binds to the ATP-binding pocket in CDK5: the chromone ring of myricetin forms an hydrogen bond with Cys83 in the hinge region of CDK5; the 3′, 4′, 5′-trihydroxyphenyl group of myricetin forms a pair of hydrogen bonds with the side chain of Asp86 and the backbone of Asp84 in CDK5; the 7-hydroxy group of myricetin interacts with the side chain of Asp144 and Lys33.
A decrease in total and nuclear levels of PDX1, a transcription factor that plays an essential role in β-cell survival, has been reported under hyperglycemic conditions [2122]. CDK5 inhibition induces the restoration of the nuclear levels of PDX1 by preventing its translocation from the nucleus to the cytosol [10]. Thus, we evaluated the effects of myricetin on PDX1 in HG-exposed cells. HG exposure reduced both the mRNA and protein expression of PDX1 and these changes were attenuated by myricetin (Fig. 4A and B). Similarly, roscovitine treatment reversed the downregulation of PDX1 induced by HG (Fig. 4C). In addition, immunofluorescence studies in INS-1 cells showed that myricetin increase the nuclear accumulation of PDX1 in HG conditions (Fig. 4D).
A recent study demonstrated that loss of PDX1 results in decreased expressions of SERCA2b, leading to a reduction in ER Ca2+ concentration and subsequent induction of ER stress [23]. Thus, we examined the effects of myricetin on the expression of SERCA2b and ER stress in HG-exposed cells. HG exposure reduced the mRNA and protein levels of SERCA2b and these changes were significantly reversed by treatment with myricetin (Fig. 5A and B). As shown in Fig. 5C, myricetin treatment significantly attenuated the HG-induced increase in the expressions of ER stress markers including P-PERK, P-eIF2α, ATF4, and CHOP and reversed the HG repressed GRP78. JNK phosphorylation was also inhibited by myricetin (Fig. 5D).
Because myricetin increased SERCA2b expression, we next evaluated whether myricetin can prevent ER stress and apoptosis, which are induced by SERCA inhibition. Treatment with thapsigargin (TG), a potent inhibitor of SERCA, increased the expression of ER stress markers (Supplementary Fig. 1A). This TG-induced ER stress response was significantly reversed by myricetin treatment. Moreover, treatment with TG increased mitochondrial potential loss Δψm and reduced Bcl-2 expression, increased the release of cytochrome c into cytosol, and the expression of cleaved caspase-3, resulting in increased apoptosis (Supplementary Fig. 1B–D). All these changes, caused by TG, were significantly reversed by myricetin.
To confirm, in a more functional model, the potentiating effect of myricetin on glucose-stimulated insulin secretion (GSIS) was analyzed in INS-1 cells. First, to define the effects of myricetin on insulin expression in this model, INS-1 cells were exposed to HG (30 mM) with and without myricetin (20 µM) for 24 hours. We found a decrease in insulin mRNA at 24 hours by HG. Co-incubation with myricetin significantly increased insulin mRNA (Fig. 6A). On the other hand, myricetin potentiated HG (16.6 mM)-induced insulin secretion, but no effect on low glucose (Fig. 6B).
Pancreatic β-cell apoptosis is a key event contributing to the pathogenesis of T2DM, and hyperglycemia plays a pivotal role in β-cell apoptosis [23]. Thus, the development of strategies aimed at preventing β-cell apoptosis is critical for the treatment of T2DM. Myricetin is a natural flavonoid that exerts therapeutic effects against T2DM [141516]. Dietary intervention studies have identified that flavonoids as dietary biomarkers in clinical trials [24]. On the other hand, myricetin could also affect inflammatory signal pathways in lipopolysaccharide-stimulated primary macrophages and RAW264.7 macrophages via the down-regulation of transcription factor nuclear factor κB binding activity [25]. Myricetin was also reported to inhibit interleukin-1β-induced inflammation in human synovial sarcoma cells [26]. Moreover, several studies determined the protective effect of myricetin against 1-methyl-4-phenylpyridinium-induced cell death by increasing the Bcl-2/Bax ratio, and inhibit the activation of caspase-3 [27]. However, whether myricetin has protective effects against hyperglycemia-induced β-cell apoptosis has yet to be evaluated. In the present study, we demonstrated that treatment with myricetin protects β-cells against HG-induced apoptosis by inhibiting ER stress, possibly through the inactivation of CDK5.
Mitochondrial dysfunction is a central contributor to β-cell apoptosis, and is characterized by elevated ROS levels and enhanced mitochondrial potential (Δψm) loss [19]. In the present study, we found that treatment with myricetin ameliorated HG-induced mitochondrial dysfunction in INS-1 cells. Mitochondrial dysfunction induces the release of cytochrome c from the mitochondria to the cytosol, where it promotes the assembly of the apoptosome, which results in caspase-9 activation and subsequent activation of the executioner caspases, including caspase-3 [28]. In addition, the Bcl-2 family of proteins, including pro-apoptotic (such as Bax) and anti-apoptotic (such as Bcl-2) proteins, represent a critical intracellular checkpoint in mitochondria-dependent apoptosis. In the present study, the treatment with myricetin attenuated release of cytochrome c and activation of caspase-3 in HG-exposed cells. Furthermore, myricetin treatment decreased Bax expression and increased Bcl-2 expression. These results suggest that myricetin prevents the β-cell apoptosis induced by HG, at least in part, by inhibiting the mitochondria-dependent apoptotic pathway.
CDK5 inhibition has been suggested as a potential therapeutic strategy for the treatment of T2DM [6]. A previous study, by performing in vitro kinase assays, showed the inhibitory effects of myricetin on the activity of CDK5 [20]. Therefore, we speculated that CDK5 may be a molecular target for the protective effects of myricetin from HG-induced β-cell apoptosis. We found that chronic exposure of INS-1 cells to HG increased the phosphorylation of CDK5 on Tyr15, but did not affect total CDK5 expression. Because CDK5 activity has been reported to be stimulated by Tyr15 phosphorylation [29], these results indicate that HG exposure activates CDK5 in β-cells. Moreover, p35, an activator of CDK5, was upregulated in HG conditions. Consistent with our data, Ubeda et al. [10] previously showed that HG exposure activates CDK5 by increasing the expression of p35 without changing the levels of total CDK5 protein in β-cells. Furthermore, the proposed binding model of myricetin to CDK5, based on docking studies, showed that myricetin binds to the ATP-binding pocket of CDK5 in an ATP-competitive manner, indicating that myricetin may inhibit the activity of CDK5 by interfering with the ATP binding site of CDK5. Taken together, these results suggest that myricetin could suppress CDK5 activity in HG-exposed β-cells at several different levels including the suppression of CDK5 phosphorylation, downregulation of p35, and direct binding to CDK5. Although more studies are required to clarify the molecular mechanisms underlying the effect of myricetin, our findings suggest CDK5 as a potential molecular target of myricetin.
PDX1 is a transcription factor with critical roles in β-cell survival [21]. Under hyperglycemic conditions, both the total levels and the nuclear levels of PDX1 decrease [1022]. In addition, CDK5 inhibition restores the reduced nuclear levels of PDX1 by inhibiting the translocation of PDX1 from the nucleus to the cytosol [10]. Consistent with these previous studies, we found that CDK5 inhibition by roscovitine increased the nuclear levels of PDX1 in HG-exposed INS-1 cells. In addition, in cells exposed to HG, roscovitine restored total PDX1 levels. Similar results were obtained upon treatment of HG-exposed cells with myricetin. These results suggest that CDK5 inhibition by myricetin may be responsible for the increased total PDX1 level and the enhanced nuclear localization of PDX1 in β-cells. Recently, SERCA2b was identified as a novel transcriptional target of PDX1 [23]. SERCA pumps cytosolic Ca2+ into the ER lumen; hence, inhibition of SERCA leads to depletion of ER Ca2+. Johnson et al. [23] showed that loss of PDX1 resulted in decreased expression of SERCA2b, leading to the reduction in ER Ca2+ concentration and the subsequent induction of ER stress. In the present study, we showed that HG exposure resulted in a reduction in SERCA2b expression, and an elevation in ER stress markers and JNK phosphorylation. Roscovitine and myricetin counteracted the reduction of SERCA2b level in HG conditions. Moreover, myricetin treatment attenuated the increase in the expressions of ER stress markers and P-JNK induced by HG. Thus, the CDK5-PDX1-SERCA2b axis may be involved in the suppressive effects of myricetin against HG-induced ER stress.
Accumulating evidence suggests that ER stress is an important contributor to β-cell apoptosis [3]. It has been shown that the interaction between ER and mitochondria regulates Ca2+-dependent cellular processes such as apoptosis. Chronic ER stress causes the release of Ca2+ from the ER to the cytosol, leading to increased Ca2+ uptake into the mitochondrial matrix. ER stress-induced accumulation of mitochondrial Ca2+ triggers mitochondrial dysfunction and the subsequent activation of the mitochondria-dependent apoptotic pathway [30]. In the present study, we found that ER stress and apoptosis induced by SERCA inhibition were significantly attenuated by myricetin. These results suggest that the suppression of ER stress by myricetin, possibly through the increase of SERCA2b expression, contributes to its protective effects against mitochondria-dependent apoptosis. Moreover, in our studies, myricetin treatment also attenuate the inhibitory effect of HG on insulin mRNA through increasing PDX1 expression and potentiates GSIS.
In conclusion, myricetin protects β-cells against HG-induced apoptosis by attenuating ER stress, possibly through the inactivation of CDK5 and consequent upregulation of PDX1 and SERCA2b. Our results provide new evidence for the mechanism of action of myricetin and strengthen the concept that myricetin is a potential therapeutic agent for the treatment of T2DM.
Figures and Tables
Fig. 1
Myricetin protects INS-1 cells and isolated rat islets from high glucose (HG)-induced apoptosis. (A) Chemical structure of myricetin: carbon numbering is indicated. (B) INS-1 cells were treated with the indicated concentrations of myricetin for 24 hours. Cell viability was measured using the Cell Counting Kit-8 (Dojindo Laboratories). (C, D) INS-1 cells (C) and isolated rat islets (D) were incubated with 30 mM glucose (HG) in the presence or absence of the indicated concentrations of myricetin for 24 hours (C) or 48 hours (D), respectively. Cell apoptosis was assessed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. All data are expressed as the mean±standard deviation of at least three independent experiments. aP<0.001 vs. control, bP<0.005 vs. HG, cP<0.05 vs. HG.

Fig. 2
Myricetin attenuates mitochondrial dysfunction in INS-1 cells exposed to high glucose (HG). (A–D) INS-1 cells were incubated with 30 mM glucose (HG) in the presence or absence of myricetin for 24 hours. (A) Intracellular reactive oxygen species (ROS) production was measured using 2′, 7′-dichlorodihydrofluorescein diacetate (DCF-DA). Data are expressed as the mean±standard deviation of at least three independent experiments. (B) Representative flow cytometry analysis images of the mitochondrial membrane potential observed with the 3,3′-dihexyloxacarbocyanine iodide (DiOC6) dye. (C) Representative image of Western blot analysis of cytochrome c in cytosol and cleaved caspase-3 (C-caspase 3). (D) Representative images of Western blot analysis of Bax/B-cell lymphoma 2 (Bcl-2). aP<0.01 vs. control, bP<0.05 vs. HG, cP<0.05 vs. control, dP<0.001 vs. control, eP<0.001 vs. HG.
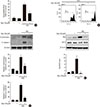
Fig. 3
Myricetin inhibits cyclin-dependent kinase 5 (CDK5) in high glucose (HG)-exposed INS-1 cells. (A) INS-1 cells were incubated with 30 mM glucose (HG) in the presence or absence of roscovitine for 24 hours. Cell apoptosis was assessed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. Data are expressed as the mean±standard deviation of at least three independent experiments. (B) INS-1 cells were incubated with 30 mM glucose (HG) with myricetin or roscovitine for 24 hours. Representative images of western blot analysis of cleaved caspase-3. (C) INS-1 cells were incubated with 30 mM glucose with or without myricetin for different time periods. Representative images of Western blot analysis of CDK5 phosphorylated at tyrosine 15 (Tyr15) and p35. (D) The proposed binding model of myricetin to CDK5 based on docking studies. The dotted lines indicate hydrogen bonds interactions. aP<0.01 vs. control, bP<0.05 vs. HG, cP<0.001 vs. control, dP<0.005 vs. HG, eP<0.001 vs. HG, fP<0.05 vs. control.
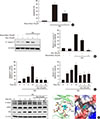
Fig. 4
Myricetin counteracts the decrease in total and nuclear levels of pancreatic duodenal homeobox 1 (PDX1) in high glucose (HG)-exposed INS-1 cells. (A, B) INS-1 cells were incubated with 30 mM glucose (HG) in the presence or absence of myricetin for 24 hours. (A) PDX1 mRNA levels were determined by real-time polymerase chain reaction (PCR). Data are expressed as the mean±standard deviation of at least three independent experiments. (B) Representative images of Western blot analysis of PDX1. (C) INS-1 cells were incubated with 30 mM glucose (HG) in the presence or absence of roscovitine for 24 hours. Representative images of western blot analysis of PDX1. (D) INS-1 cells were incubated with 30 mM glucose (HG) along with myricetin for 24 hours. Representative images of the subcellular localization of PDX1 by confocal microscope. 4′,6-Ddiamidino-2-phenylindole (DAPI) was used to stain the nuclei. aP<0.05 vs. control, bP<0.001 vs. control, cP<0.05 vs. HG, dP<0.01 vs. HG, eP<0.01 vs. control.
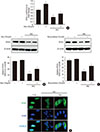
Fig. 5
Myricetin conterbalances the decrease in sarcoendoplasmic reticulum calcium ATPase 2b (SERCA2b) expression and prevents endoplasmic reticulum (ER) stress in INS-1 cells exposed to high glucose (HG). (A, B) INS-1 cells were incubated with 30 mM glucose (HG) in the presence or absence of myricetin for 24 hours. (A) SERCA2b mRNA levels were determined by real-time polymerase chain reaction (PCR). Data are expressed as the mean±standard deviation of at least three independent experiments. (B) INS-1 cells were incubated with 30 mM glucose (HG) with myricetin for 24 hours. Representative images of Western blot analysis of SERCA2b. (C, D) INS-1 cells were incubated with 30 mM glucose (HG) in the presence or absence of myricetin for 24 hours. Representative images of Western blot analysis of (C) ER stress markers: glucose regulated protein 78 (Grp78), phosphorylated protein kinase R-like endoplasmic reticulum kinase (P-PERK), phosphorylated eukaryotic initiation factor 2α (P-eIF2α), activating transcription factor 4 (ATF4), and CCAAT-enhancer-binding protein homologous protein (CHOP). (D) Phosphorylated c-Jun N-terminal kinase (P-JNK). aP<0.05 vs. control, bP<0.05 vs. HG, cP<0.01 vs. control, dP<0.005 vs. control, eP<0.005 vs. HG, fP<0.01 vs. HG, gP<0.001 vs. control, hP<0.001 vs. HG.

Fig. 6
Myricetin effect on insulin mRNA and glucose-stimulated insulin secretion (GSIS). (A) INS-1 cells were incubated with 30 mM glucose (high glucose [HG]) in the presence or absence of myricetin for 24 hours and insulin mRNA levels was determined by real-time polymerase chain reaction (PCR). Data are expressed as the mean±standard deviation of at least three independent experiments. (B) GSIS was measured by rat insulin radioimmunoassay as described in the methods section. aP<0.001 vs. control, bP<0.05 vs. control, cP<0.05 vs. HG, dP<0.05 vs. 16.6 mM glucose control.

ACKNOWLEDGMENTS
This work was supported by the Kyungpook National University Bokhyeon Research Fund, 2015 (to In-Kyu Lee), and by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (grant number 2016R1E1A2020567) (to Jaechan Leem).
References
1. Leahy JL. Pathogenesis of type 2 diabetes mellitus. Arch Med Res. 2005; 36:197–209.
2. Poitout V, Robertson RP. Minireview: secondary beta-cell failure in type 2 diabetes. A convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002; 143:339–342.
3. Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol Metab. 2011; 22:266–274.
4. Karunakaran U, Kim HJ, Kim JY, Lee IK. Guards and culprits in the endoplasmic reticulum: glucolipotoxicity and β-cell failure in type II diabetes. Exp Diabetes Res. 2012; 2012:639762.
5. Hara T, Mahadevan J, Kanekura K, Hara M, Lu S, Urano F. Calcium efflux from the endoplasmic reticulum leads to β-cell death. Endocrinology. 2014; 155:758–768.
6. Wei FY, Tomizawa K. Cyclin-dependent kinase 5 (Cdk5): a potential therapeutic target for the treatment of neurodegenerative diseases and diabetes mellitus. Mini Rev Med Chem. 2007; 7:1070–1074.
7. Weishaupt JH, Kussmaul L, Grotsch P, Heckel A, Rohde G, Romig H, Bahr M, Gillardon F. Inhibition of CDK5 is protective in necrotic and apoptotic paradigms of neuronal cell death and prevents mitochondrial dysfunction. Mol Cell Neurosci. 2003; 24:489–502.
8. Sun KH, de Pablo Y, Vincent F, Shah K. Deregulated Cdk5 promotes oxidative stress and mitochondrial dysfunction. J Neurochem. 2008; 107:265–278.
9. Ubeda M, Kemp DM, Habener JF. Glucose-induced expression of the cyclin-dependent protein kinase 5 activator p35 involved in Alzheimer’s disease regulates insulin gene transcription in pancreatic beta-cells. Endocrinology. 2004; 145:3023–3031.
10. Ubeda M, Rukstalis JM, Habener JF. Inhibition of cyclin-dependent kinase 5 activity protects pancreatic beta cells from glucotoxicity. J Biol Chem. 2006; 281:28858–28864.
11. Wei FY, Nagashima K, Ohshima T, Saheki Y, Lu YF, Matsushita M, Yamada Y, Mikoshiba K, Seino Y, Matsui H, Tomizawa K. Cdk5-dependent regulation of glucose-stimulated insulin secretion. Nat Med. 2005; 11:1104–1108.
12. Zheng YL, Hu YF, Zhang A, Wang W, Li B, Amin N, Grant P, Pant HC. Overexpression of p35 in Min6 pancreatic beta cells induces a stressed neuron-like apoptosis. J Neurol Sci. 2010; 299:101–107.
13. Semwal DK, Semwal RB, Combrinck S, Viljoen A. Myricetin: a dietary molecule with diverse biological activities. Nutrients. 2016; 8:90.
14. Liu IM, Tzeng TF, Liou SS, Lan TW. Myricetin, a naturally occurring flavonol, ameliorates insulin resistance induced by a high-fructose diet in rats. Life Sci. 2007; 81:1479–1488.
15. Liu IM, Tzeng TF, Liou SS, Lan TW. Improvement of insulin sensitivity in obese Zucker rats by myricetin extracted from Abelmoschus moschatus. Planta Med. 2007; 73:1054–1060.
16. Choi HN, Kang MJ, Lee SJ, Kim JI. Ameliorative effect of myricetin on insulin resistance in mice fed a high-fat, high-sucrose diet. Nutr Res Pract. 2014; 8:544–549.
17. Ding Y, Zhang ZF, Dai XQ, Li Y. Myricetin protects against cytokine-induced cell death in RIN-m5f β cells. J Med Food. 2012; 15:733–740.
18. Park KG, Lee KM, Seo HY, Suh JH, Kim HS, Wang L, Won KC, Lee HW, Park JY, Lee KU, Kim JG, Kim BW, Choi HS, Lee IK. Glucotoxicity in the INS-1 rat insulinoma cell line is mediated by the orphan nuclear receptor small heterodimer partner. Diabetes. 2007; 56:431–437.
19. Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010; 12:537–577.
20. Zapata-Torres G, Opazo F, Salgado C, Munoz JP, Krautwurst H, Mascayano C, Sepulveda-Boza S, Maccioni RB, Cassels BK. Effects of natural flavones and flavonols on the kinase activity of Cdk5. J Nat Prod. 2004; 67:416–420.
21. Fujimoto K, Polonsky KS. Pdx1 and other factors that regulate pancreatic beta-cell survival. Diabetes Obes Metab. 2009; 11:Suppl 4. 30–37.
22. Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest. 2013; 123:3305–3316.
23. Johnson JS, Kono T, Tong X, Yamamoto WR, Zarain-Herzberg A, Merrins MJ, Satin LS, Gilon P, Evans-Molina C. Pancreatic and duodenal homeobox protein 1 (Pdx-1) maintains endoplasmic reticulum calcium levels through transcriptional regulation of sarco-endoplasmic reticulum calcium ATPase 2b (SERCA2b) in the islet β cell. J Biol Chem. 2014; 289:32798–32810.
24. Noroozi M, Burns J, Crozier A, Kelly IE, Lean ME. Prediction of dietary flavonol consumption from fasting plasma concentration or urinary excretion. Eur J Clin Nutr. 2000; 54:143–149.
25. Kang BY, Kim SH, Cho D, Kim TS. Inhibition of interleukin-12 production in mouse macrophages via decreased nuclear factor-kappaB DNA binding activity by myricetin, a naturally occurring flavonoid. Arch Pharm Res. 2005; 28:274–279.
26. Lee YS, Choi EM. Myricetin inhibits IL-1beta-induced inflammatory mediators in SW982 human synovial sarcoma cells. Int Immunopharmacol. 2010; 10:812–814.
27. Zhang K, Ma Z, Wang J, Xie A, Xie J. Myricetin attenuated MPP(+)-induced cytotoxicity by anti-oxidation and inhibition of MKK4 and JNK activation in MES23.5 cells. Neuropharmacology. 2011; 61:329–335.
28. Bhola PD, Letai A. Mitochondria-judges and executioners of cell death sentences. Mol Cell. 2016; 61:695–704.
29. Zukerberg LR, Patrick GN, Nikolic M, Humbert S, Wu CL, Lanier LM, Gertler FB, Vidal M, Van Etten RA, Tsai LH. Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron. 2000; 26:633–646.
30. Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008; 27:6407–6418.
SUPPLEMENTARY MATERIAL
Supplementary Fig. 1
Myricetin attenuates endoplasmic reticulum (ER) stress induced by sarcoendoplasmic reticulum calcium ATPase (SERCA) inhibition and apoptosis in INS-1 cells. (A–D) INS-1 cells were incubated with 0.5 µM thapsigargin (TG) in the presence or absence of the indicated concentrations of myricetin for 24 hours. (A) Representative images of Western blot analysis of ER stress markers: glucose regulated protein 78 (Grp78), phosphorylated protein kinase R-like endoplasmic reticulum kinase (P-PERK), phosphorylated eukaryotic initiation factor 2α (P-eIF2α), activating transcription factor 4 (ATF4), and CCAAT-enhancer-binding protein homologous protein (CHOP). (B) Representative flow cytometry analysis images of mitochondrial membrane potential observed with 3,3′-dihexyloxacarbocyanine iodide (DiOC6) dye. (C) Representative images of Western blot analysis of cytochrome c in the cytosol, cleaved caspase-3 (C-Capase 3), and B-cell lymphoma 2 (Bcl-2). (D) Cell apoptosis was assessed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. All data are expressed as the mean±standard deviation of at least three independent experiments. aP<0.05 vs. control, bP<0.001 vs. control, cP<0.01 vs. TG, dP<0.001 vs. TG, eP<0.01 vs. control, fP<0.05 vs. TG, gP<0.005 vs. TG.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download