Introduction
Peripheral artery disease (PAD) is generally a manifestation of advanced systemic atherosclerosis. Risk factors strongly associated with the development of PAD include older age, diabetes mellitus (DM), smoking, and male sex.
1)2)3) Patients with PAD often have coexisting coronary artery disease (CAD) or cerebral artery disease.
4) In general, the presence of PAD is associated with poor prognosis as well as relatively high mortality and morbidity rates.
5)
Patients with PAD in the lower extremity may be asymptomatic or may present with symptoms ranging from intermittent claudication, to rest pain, non-healing sores and even gangrene. For moderate to severe symptoms, endovascular or surgical revascularization of diseased arteries is indicated.
1)2)3) Endovascular treatment has been traditionally recommended for shorter-duration, limited lesions, whereas surgical options have been chosen for extensive and more complex lesions. However, with the development of newer devices and techniques for endovascular therapy, more complex lesions are increasingly being treated with endovascular options due to their advantages over surgical treatments, including less invasiveness, fewer complications, earlier recovery and shorter hospitalization.
A recent study using Korean Health Insurance data reported the frequency of endovascular treatment in the lower extremity increased more than two-fold from 2004 to 2013, whereas open surgery decreased by 39% over the same period.
6) However, knowledge regarding current practice patterns and outcomes of endovascular therapy in Korea is limited. In the present study, baseline clinical and lesion characteristics of Korean patients with lower extremity artery disease who were treated with endovascular methods were investigated.
Discussion
The prevalence of lower extremity artery disease in Korea remains unknown; however, with progressive aging of the Korean population, the number of patients with PAD undergoing endovascular or surgical treatment is rapidly increasing. According to a recent study based on National Health Insurance data, the total number of procedures increased each year from 2008 to 2012 by 20 to 25%.
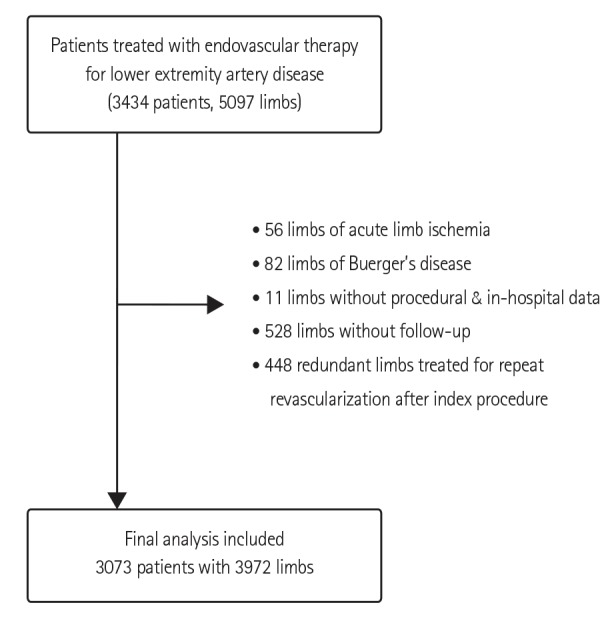
9) Among the total procedures performed for treatment of lower extremity artery diseases, 80 to 95% are endovascular treatments. Furthermore, the number of endovascular treatment centers increased from 120 in 2008 to 163 in 2012. However, to date, minimal representative data exist regarding the characteristics and outcomes of patients undergoing endovascular treatment for lower extremity artery disease in Korea. Thus, we established a Korean multicenter registry containing a retrospective and prospective cohort to address this issue. The present study is the first report on the baseline characteristics of the retrospective cohort.
Male sex, older age, DM, smoking, hypertension, and hypercholesterolemia are established risk factors for atherosclerosis and PAD.
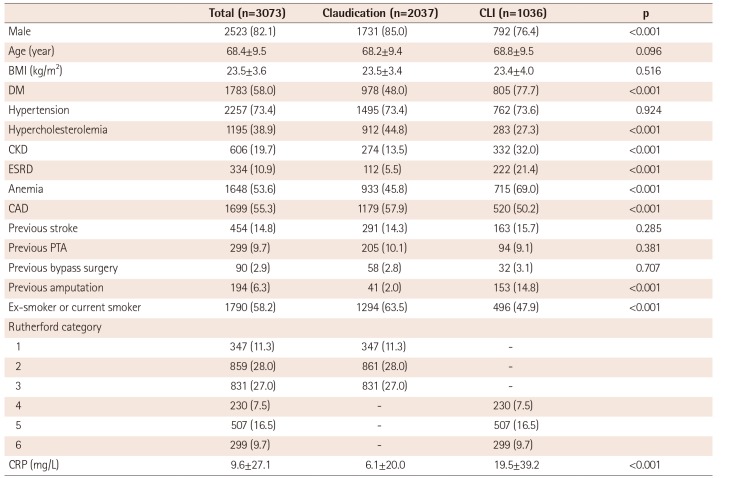
1)2)3) In our study cohort, the mean age was 68 years, and the majority of patients were male (82.1%) and current smokers (58.2%) with DM (58.0%) and hypertension (73.4%). Atherosclerotic diseases, such as CAD (55.3%), stroke (14.8%), and CKD (19.7%), frequently coexisted with PAD in our patients. Approximately two-thirds of patients presented with intermittent claudication and one-third with CLI. In previous studies, chronic heavy smoking, older age, DM, and CKD were identified as risk factors for CLI.
1)10)11) Similarly, we found DM and CKD, including ESRD, were more common in the CLI group than in the claudication group. However, no significant difference in age was observed between the claudication and CLI groups. Male sex, hypercholesterolemia, and smoking were less frequent in the CLI group than the claudication group. Thus, in our patient cohort, DM and CKD were the most important factors in the development of CLI. In particular, DM was present in 77% of the CLI patients. This prevalence of DM in CLI patients is similar to that reported in Japanese patients with CLI (68 to 71%)
12)13) but higher than the prevalence in European or American patients with CLI (44.1 to 61%).
14)15)16)17) Conversely, the prevalence of CKD (32%) among CLI patients in our study was lower than in the Japanese (61%)
12) or European study cohorts (57%) of patients with CLI.
15)
The basic pathophysiology of CLI involves macro- and microvascular defects that lead to reduced arterial perfusion insufficient to meet the resting tissue demands for oxygen and nutrients. Endothelial damage caused by inflammation and oxygen radicals, inappropriate platelet aggregation, tissue edema, and inadequate angiogenesis are hypothesized to contribute to the development of CLI.
11) DM and CKD are two representative diseases with increased level of systemic inflammation and an excessive burden of oxygen radicals. Patients with DM and renal dysfunction typically have diffuse, severe infrapopliteal arterial occlusive disease. Patients with CKD, and especially those with ESRD, are prone to developing medial calcifications, which are commonly observed in the tibial and femoral arteries. Severe vascular calcifications are the major obstacles to endovascular treatment.
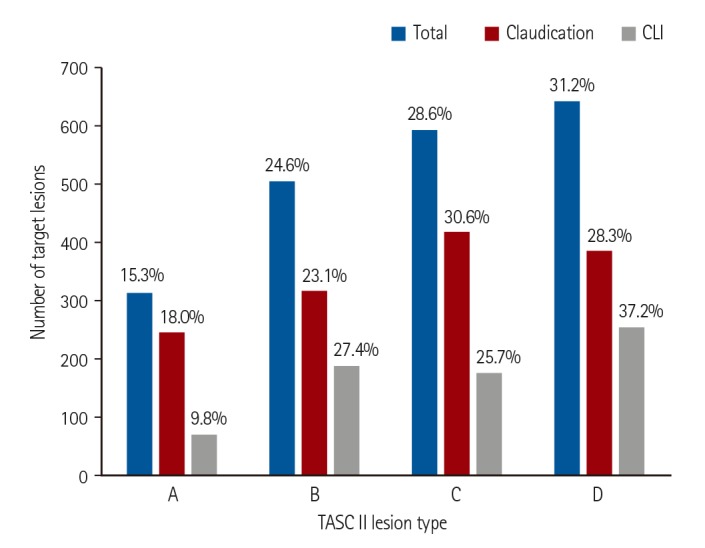
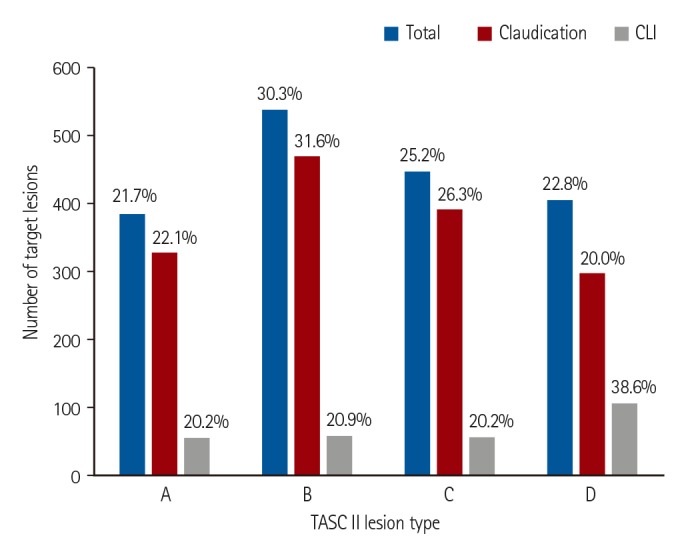
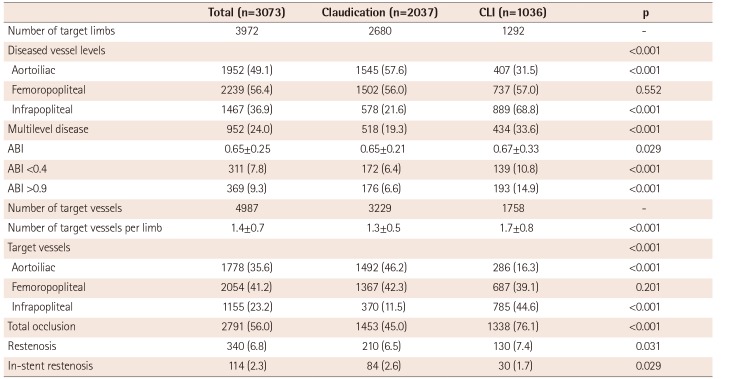
In the present study, the femoropopliteal artery was the most common target of endovascular treatment for the entire study cohort. However, the aortoiliac arteries were the most common target vessels in the claudication group, whereas infrapopliteal arteries were most commonly treated in the CLI group. Complex lesions, such as TASC II type D lesions, were frequently treated with endovascular therapy. Among the femoropopliteal artery lesions, type D lesions were the most common targets (31.2%). Among the aortoiliac lesions, 22.8% were type D lesions. International guidelines primarily recommend surgery as the treatment of choice for type D aortoiliac or femoropopliteal lesions.
1)2)3) European guidelines suggest that type D lesions can be treated by endovascular procedures in patients with severe comorbidities if they are performed by an experienced team.
2) There may be several reasons for the preferential use of endovascular therapy for type D lesions observed in our cohort. First, patient factors may have played a role. Patients requiring treatment were frequently old and had comorbidities such as CAD, previous stroke, and CKD. Often, patients preferred to undergo less invasive procedures. Second, physician factors may have also played a role. This is a retrospective cohort of patients treated by interventional cardiologists. Therefore, physicians tended to treat complex lesions primarily with endovascular techniques.
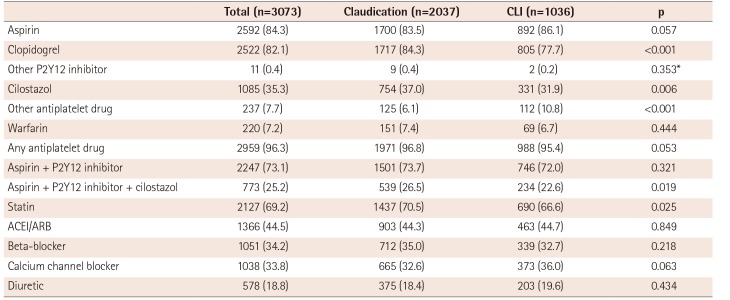
In general, there were relatively high prescription rates for antiplatelet drugs after endovascular therapy in both the claudication and CLI groups. Nevertheless, only 73.1% of patients received combination antiplatelet therapy consisting of aspirin plus a P2Y12 inhibitor and only 69.2% of patients received a statin. Dual antiplatelet therapy with aspirin and a thienopyridine is generally recommended for at least 1 month after endovascular therapy, especially when performed with stent implantation.
2)3) In the present study, the rate of antiplatelet therapy using at least 1 antiplatelet drug was high (96.3%); however, the 73.1% rate of dual antiplatelet therapy with aspirin plus a P2Y12 inhibitor at hospital discharge was lower than expected. In patients with PAD, guidelines recommend lipid-lowering therapy with a low-density lipoprotein (LDL) cholesterol goal of <70 mg/dL and/or a ≤50% reduction in LDL cholesterol.
2)3) Previous studies demonstrated benefits of statins in reducing mortality and cardiovascular events in patients with PAD.
18)19)20) Thus, the statin prescription rate of approximately 70% at hospital discharge was disappointing. The statin prescription rate was even lower (66.6%) in patients with CLI. Therefore, approximately 30% of patients in our cohort did not receive optimal post-procedural medical treatment to prevent adverse cardiovascular events. There may have been various reasons for not prescribing adequate antiplatelet agents or statins. However, the reasons could not be clarified within the limits of this retrospective study. This rate of non-adherence to guidelines for optimal medical treatment is high and alarming. Physicians' awareness of this issue should be increased as well as the effort in providing optimal treatment to prevent adverse cardiovascular events.
This study had several limitations. First, due to the retrospective study design, inherent limitations existed. Second, endovascular treatment was performed in this registry study by interventional cardiologists. Therefore, the study may not represent patient characteristics or practice patterns of endovascular practitioners of other specialties, such as interventional radiologists or vascular surgeons. Third, the lesion characteristics were not analyzed in a central imaging core laboratory.
In conclusion, the majority of Korean patients with PAD exhibited conventional risk factors, such as male sex, older age, DM, and hypertension with coexisting CAD. Complex lesions were frequently treated with endovascular therapy. However, the rate of adherence to guidelines regarding post-procedural medical treatment should be improved in the future.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download