Abstract
Background and Objectives
Cardiovascular complications are the leading cause of morbidity and mortality in childhood cancer survivors. Hematopoietic stem cell transplantation (HSCT) is a curable therapy for pediatric cancer. However, changes in cardiac function in children after HSCT are not well known. We assessed left ventricular (LV) function in children after HSCT using speckle tracking echocardiography (STE).
Subjects and Methods
Forty consecutive patients with median age of 11.9 years (range, 1.5-16 years) who received HSCT for acute leukemia and had comprehensive echocardiography before and after (median 9.2 month) HSCT were included in this study. The LV function parameters including conventional tissue Doppler imaging (TDI) and STE data were collected from pre- and post-HSCT echocardiography. These data were compared to those of 39 age-matched normal controls.
Results
Compared to normal controls, post HSCT patients had similar (p=0.06) LV ejection fraction. However, the following three LV function parameters were significantly decreased in post HSCT patients: rate-corrected velocity of circumferential fiber shortening (p=0.04), mitral inflow E velocity (p<0.001), and mitral septal annular E' velocity (p=0.03). The following four STE parameters were also significantly decreased in post HSCT patients: LV global circumferential systolic strain (p<0.01), strain rate (SR, p=0.01), circumferential diastolic SR (p<0.01), and longitudinal diastolic SR (p<0.001). There was no significant change in TDI or STE parameters after HSCT compared to pre-HSCT. Patients with anthracycline cumulative dose >400 mg/m2 showed significantly (p<0.05) lower circumferential systolic strain and circumferential diastolic SR.
In recent decades, survival from childhood cancer has improved dramatically due to improved supportive care, more precise risk stratification and personalized therapeutic approach, and extended application of allogeneic hematopoietic stem cell transplantation (HSCT) as life-saving therapy.1)2) However, mortality rates in childhood cancer survivors are continuously increased compared to general populations. The increase in mortality rates in childhood cancer survivors is associated with long-term organ damage and dysfunction.3)4)5)
Except for primary or secondary malignant conditions, late cardiac complications have been reported to be the leading cause of morbidity and mortality following 5-year of survival.3)4)6)7) Cardiac complications may occur within one year after treatment. They may develop insidiously over years or decades in some patients. They can manifest as subclinical myocardial dysfunction or dilated cardiomyopathy with overt heart failure.8)9) Anthracyclines, widely used for childhood malignancies, are well documented cardiotoxic agents. It is also well recognized that the risk of congestive heart failure (CHF) caused by anthracycline exposure is dose-dependent in patients not undergoing HSCT. Left ventricular (LV) dysfunction and end-stage cardiomyopathy may occur even at lower doses in some patients.5)8)9)
Hematopoietic stem cell transplantation is a life-saving therapy for pediatric cancers including acute leukemia. Indications for HSCT are expanding. Population of childhood cancer survivors who had HSCT is increasing.1) Incidence and risk factors of CHF in patients who had HSCT are not well known. Patients who had HSCT might be at risk of further cardiac injury by treatment for pre-HSCT conditioning therapies including high-dose cyclophosphamide (CY) and total body irradiation (TBI).10)11)12) However, there have been debates on the effect of pre-HSCT conditioning therapies on cardiovascular system. Recent studies showed that the risk of late cardiovascular complications after HSCT was largely due to pre-HSCT therapeutic exposures with little additional risk from conditioning related exposures.13)14) A study on the change of LV function before and after HSCT using echocardiography parameters demonstrated that children at 1-year post HSCT had subclinical declines in systolic and diastolic function.15) The authors suggested that HSCT conditioning therapies were likely to be other risk factors for LV dysfunction after HSCT. They recommended that serial assessment of cardiac function should be considered for all children following HSCT.15)
Echocardiography remains the main imaging modality to monitor cardiac function in these patients. LV ejection fraction (EF) or fractional shortening (FS), parameters of systolic function measured by conventional M-mode imaging, have been commonly included in long term follow up protocols. However, the need for geometric assumption and variable reproducibility are the limitations.16)17) Recently, studies using tissue Doppler imaging (TDI) and/or two-dimensional (2D) speckle-tracking echocardiography (STE) parameters of myocardial deformation have demonstrated that subclinical LV systolic and diastolic dysfunctions were not uncommon in long-term survivors with normal EF.18)19)20)21) In this study, we compared the LV systolic and diastolic function in children who had HSCT for acute leukemia to those of age matched normal controls using TDI and 2D STE. In addition, we examined the change of cardiac function from pre-HSCT to post-HSCT echocardiographic findings to determine the impact of HSCT on cardiac function.
This study was a single center, retrospective, and case control study in 40 children (age <18 years) with acute leukemia who had undergone the first HSCT at Seoul Saint Mary's Hospital between 1 January 2011 and 31 January 2014. According to our institutional protocol, all patients had echocardiography to assess systolic and diastolic function within 3 months before HSCT, and 3, 6, 9, 12 months and annually thereafter. The inclusion criteria were: 1) patients who had a pre-HSCT echocardiography within 3 months before HSCT and a post-HSCT echocardiography performed at least more than 3 months following HSCT, 2) patients whose echocardiographic TDI (mitral septal annular velocity) and STE {LV longitudinal and mid-LV circumferential strain (S), and strain rate (SR)} data were available. Exclusion criteria were: 1) patients who had congenital heart disease, 2) patients who had risk factors for cardiovascular complications such as hypertension, diabetes, or hyperlipidemia, 3) patients who died within 6 months after HSCT. The echocardiographic findings of patients were compared to those of 39 age-matched normal controls. The control subjects were children who visited the cardiac outpatient clinic with heart murmur, nonspecific chest pain, palpitation, or syncope with echocardiographic findings showing definitive normal cardiac structure and functions. Patients' demographic and HSCT characteristics were collected from the database of HSCT program at our institute. Total anthracycline cumulative dose were recorded as doxorubicin cumulative dose by correcting for equivalent doses of commonly used anthracyclines based on substitution rules and hematologic toxicity.22)
Patients received a TBI or a busulfan-based myeloablative conditioning regimen. Pre-HSCT conditioning regimens for this study population included busulfan+fludarabine±antithymocyte globulin (ATG) (n=22, 55%) and TBI+CY±cytarabine or ATG (n=18, 45%). Stem cell sources included bone marrow (n=27), peripheral blood stem cells (n=12), and cord blood (n=1). The regimen for graft-versus-host disease (GVHD) prophylaxis included cyclosporine (3 mg/kg/day, intravenous initially) starting from day 1 and 4 doses of mini-dose methotrexate (5 mg/m2) at days 1, 3, 6, and 11. For patients who received a transplant from an unrelated donor, ATG was given at a total dosage of 7.5 mg/kg during a period of three days as part of the conditioning procedure.
According to the protocol of HSCT program, all patients underwent comprehensive echocardiographic assessment of systolic and diastolic function using Vivid 7 ultrasound machine (GE Medical Systems, Horten, Norway) within 3 months before HSCT, and 3, 6, 9, 12 months and annually thereafter. Echocardigraphic data were collected from two studies, a pre-HSCT study performed closest to HSCT and the latest study performed at least three months following HSCT. Measurements were performed on ultrasound machines and/or customized EchoPAC software (GE Medical Systems, Horten, Norway). Average value of echocardiographic indices from three cardiac cycles was recorded.
For conventional echocardiographic assessment, LV end-systolic and end-diastolic dimensions, LV EF, and FS were derived from M-mode assessment of mid-LV parasternal long axis view. Rate-corrected velocity of circumferential fiber shortening (Vcfc) was also derived. Mitral inflow Doppler velocities at early (E) and late (A) diastole, E/A ratio, and early E deceleration time (DT) were recorded. From TDI, mitral septal annular velocities, including peak early diastolic (E'), late diastolic (A'), peak systolic myocardial tissue velocity (S'), E'/A' ratio, and mitral septal myocardial performance index (MPI) were recorded. STE for determinations of LV strain and SR was performed using customized EchoPAC software (GE Medical Systems) to track speckles. The apical four-chamber plane was used to assess LV global longitudinal strain, longitudinal systolic SR, and longitudinal diastolic SR. The parasternal short-axis plane at the level of papillary muscle was used to assess LV global circumferential strain, circumferential systolic SR, and circumferential diastolic SR. Low intra and interobserver variability for the measurement of global longitudinal strain and SR were previously reported.23)24) All post-HSCT echocardiographic parameters in patients were compared to those of normal controls and pre-HSCT parameters.
Data were expressed as mean±standard deviation. Comparison of demographic and echocardiographic parameters between patients and controls were made using two-tailed independent-sample t-test for normally distributed data and two-tailed Mann-Whitney U test for data without normal distribution, or Fisher's exact test when appropriate. Changes in echocardiographic parameters from the pre-HSCT to the post-HSCT were compared using paired-sample t-test. To evaluate the potential effect of age, sex, body mass index (BMI), oncologic diagnosis, TBI, use of CY, acute GVHD after transplant, and cumulative dose of anthracyclin on cardiac functions (TDI and STE parameters), we used the independent-samples t-test for dichotomous variables and Pearson correlation analysis for continuous variables. Correlations between LV EF and parameters derived TDI and STE were analyzed by Pearson correlation analysis. As this was an exploratory analysis, we did not adjust parameters for multiple testing. To explore the effect of anthracycline on cardiac functions, patients were divided into two groups according to their cumulative dose of anthracycline with cutoff points of 200, 300, or 400 mg/m2. Echocardiographic parameters were compared using the independent-samples t-test. Data were analyzed using commercially available software PASW Statistics (version 18 for Windows; IBM, Armonk, NY, USA). Statistical significance was considered when p was less than 0.05.
Demographic and HSCT characteristics of the study cohort are summarized in Table 1. A total of 40 patients (30 males) who had the first HSCT for acute myeloid leukemia (AML, n=20) or acute lymphoblastic leukemia (ALL, n=20) at the age of 10.2±4.9 years (range, 1.5-16 years) met our inclusion criteria. Of the 40 patients, 18 (45%) had TBI and CY treatment for pre-transplant conditioning. The median equivalent cumulative dose of anthracycline was 293 mg/m2 (range, 30-820 mg/m2). Acute GVHD developed in 16 (40%) patients with a median of 16 days (range, 5-50 days) after HSCT. All patients were asymptomatic without need for cardiac medication. Echocardiography was done with a median of 13 days (range, 5-55 days) before and 9.2 months (range, 3-13 months) after HSCT. The 39 controls (26 males) with age of 9.3±2.77 years (p=0.07) weighed 35.8±13.0 kg (vs. 42.7±22.0 kg for patients, p=0.17) at the time of study. The BMI of patients were lower than those of controls (p=0.01).
Echocardiographic characteristics of patients and controls are summarized in Table 2. Compared to controls, patients had significantly higher heart rate (p=0.01) and decreased Vcfc (p=0.04) after HSCT. Patients had slightly lower LV EF (p=0.06) and FS (p=0.06). Patients also had slightly larger LV end-systolic dimension (p=0.06). However, all patients had LV EF (>55%) and LV FS (>0.28) within normal limits. LV EF showed significant correlation (r=0.905, p<0.001) with Vcfc in patients. Patients had significantly lower mitral inflow early diastolic E velocity (p<0.001) and E/A ratio (p<0.001) compared to normal controls. In the patient group, the mitral inflow early diastolic E velocity decreased significantly (p=0.01) after HSCT as compared to pre-transplant assessment. However, other parameters did not show any significant change from pre-HSCT to post-HSCT.
Compared to the controls, patients had significantly lower mitral septal annular E' (p=0.03) and E'/A' ratio (p<0.001) but higher A' (p<0.001). Mitral septal S' and MPI on TDI after HSCT were not significantly different between the two groups (Table 2). On 2D STE, global longitudinal systolic strain and global longitudinal systolic SR were similar between patients and controls. However, global longitudinal diastolic SR (p<0.001), circumferential systolic strain (p<0.001), circumferential systolic SR (p=0.01), and circumferential diastolic SR (p<0.001) were significantly decreased in patients after HSCT compared to controls. LV EF did now show significant correlation with any parameters derived from TDI or STE. For patients, there was no significant change in TDI or STE parameters from pre-HSCT to post-HSCT.
The potential effect of age, sex, BMI, oncologic diagnosis, TBI, use of CY, acute GVHD after transplant, and cumulative dose of anthracyclin on cardiac function were analyzed. Eighteen patients who had TBI and CY as conditioning regimens showed slightly decreased global longitudinal diastolic SR (1.70±0.46 sec-1 vs. 1.99±0.55 sec-1, p=0.078) without statistical significance from patients without TBI. LV global longitudinal diastolic SR was significantly decreased in patients who had acute GVHD after HSCT (n=16, 1.67±0.55 sec-1 vs. 1.98±0.48 sec-1, p<0.048). LV global longitudinal diastolic SR showed negative correlations with BMI (r=-0.503, p=0.001) and age at diagnosis (r=-0.612, p<0.001). Sex and diagnosis (AML vs. ALL) were not associated with LV dysfunction. Patients with lifetime cumulative dose of anthracycline more than 400 mg/m2 (n=8) had significantly lower global circumferential systolic strain (-14.7±3.56% vs. -17.3±3.13%, p=0.03) and global circumferential diastolic SR (1.31±0.45 sec-1 vs. 1.80±3.13 sec-1, p=0.015) (Fig. 1).
This is the first study to evaluate LV deformation using STE after HSCT in children with acute leukemia as a homogenous group of study cohort. Our findings in this study suggest that children who had HSCT for acute leukemia had reduced LV systolic and diastolic function based on TDI and STE, albeit having normal range of LV EF or FS compared to normal controls. Patients had small but significantly lower Vcfc (an afterload-dependent measure of contractility). In addition, patients had decreased mitral inflow Doppler E velocity and E/A ratio (an early markers of diastolic dysfunction). Mitral septal peak E' tissue Doppler velocity was significantly decreased in patients. In addition, patients had significantly reduced LV myocardial deformation parameters, circumferential strain, SR, and longitudinal SR. These results might have been significantly influenced by pre-transplant cumulative dose of anthracyclin (>400 mg/m2). The impact of pre-transplant conditioning treatment on cardiac function was minimal. All patients were asymptomatic without needing cardiac medication in our study.
Despite a dramatic increase in cure rate of childhood cancer, high long-term mortality and morbidity in this population compared to general populations have been reported to be related to long-term organ damage and dysfunction.1)2)3)5) Oeffinger et al.25) reported that 30 years after treatment, the cumulative incidence of chronic health conditions in long-term cancer survivors attained 73%, with a cumulative incidence of 42% for severe, disabling, or life threatening conditions or death. Late cardiac complications, except for patients' own malignant conditions, have been reported to be the leading cause of morbidity and mortality in childhood cancer survivors after 5-year of survival.3)6)7) Mertens4) reported that childhood cancer survivors are 15 times more likely to develop clinical heart failure with significantly increased mortality rate due to cardiac causes compared to controls. Cardiac complications can manifest as subclinical myocardial dysfunction, acute toxicity with overt heart failure early after treatment, or insidious onset dilated cardiomyopathy over years or decades after treatment.8)9) In this relatively short-term follow up study, although there was no patient with clinical heart failure needing cardiac medication, subclinical cardiac systolic and diastolic dysfunction assessed by TDI and STE was evident in patients after HSCT compared to normal controls, albeit having normal range of LV EF or FS. A study on the change of LV function before and after HSCT using echocardiography parameters also demonstrated that children had subclinical declines in systolic and diastolic function at 1-year post HSCT.15) Despite the uncertainties of the overall clinical significance, subclinical cardiac dysfunction is known to be more prevalent than symptomatic heart failure which might become clinically important over time.15)26) Serial close follow up evaluation of cardiac function should be considered for all children following HSCT.
Hematopoietic stem cell transplantation has an expanding role as a life-saving therapy for pediatric cancers, including acute leukemia. The population of childhood cancer survivors who had HSCT is increasing.1) However, the incidence and predictors of CHF in childhood cancer survivors with HSCT has not been well known, although more extensive studies have been done in non-HCT settings. Risk factors of developing cardiovascular diseases such as hypertension and diabetes has been reported to be increased in childhood HSCT survivors.25)27) A study on 1491 patients (mainly adults) who had survived 2 years or longer after HSCT showed that HSCT recipients experienced increased cardiovascular death {adjusted incidence rate difference, 3.6 per 1000 person-years (95% confidence interval, 1.7 to 5.5)}. In addition, they had an increased cumulative incidence of ischemic heart disease, cardiomyopathy or heart failure, stroke, vascular diseases, and rhythm disorders compared to the general population.3) Besides pre-HSCT chemotherapeutic exposure, especially anthracyclin derivatives that are well-known cardiotoxic agents, conditioning treatment with high-dose CY and TBI with potentially cardiotoxic exposures unique to HCT recipients may affect further cardiac injury.10)11)12)25)27) However, there has been debates on this issue regarding the impact of conditioning treatment on cardiac function.13)14)28) Chow et al.28) found that no consistent difference in hazards of cardiovascular complications was observed after TBI. In contrast, Daly et al.15) showed that LV systolic performance decreased significantly after HSCT in subgroup of patients without exposure to anthracycline chemotherapy and suggested that the decrease in LV contractility was likely to be secondary to other risk factors, such as HSCT conditioning therapy. In our patients, a small but statistically significant decrease in mitral inflow Doppler peak early diastolic E velocity after HSCT was founds, whereas other echocardiographic parameters including TDI and STE did not change significantly after HSCT compared to pre-HSCT parameters. In addition, post-HSCT echocardiographic functional parameters of 18 patients who had TBI and CY as conditioning regimens showed no significant difference from those of patients without TBI or CY. These findings suggested that the impact of preconditioning treatment on cardiac function was minimal in our patients, although longer follow up evaluation is needed to verify this.
Anthracyclines, widely used in childhood cancer treatment, are well known cardiotoxic agents. The risk of CHF caused by anthracycline has been known to be dose-dependent. Further studies have demonstrated evidence of cardiac dysfunction at a low cumulative doses of anthracycline <250 mg/m2.5)8)9) Daly et al.15) reported that children had subclinical LV systolic and diastolic dysfunction at 1-year post HSCT. However, cumulative lifetime anthracycline dose did not correlate with observed changes in function. In our study, patients with cumulative doses of anthracycline >400 mg/m2 had significantly lower LV deformation parameters such as global circumferential systolic strain (p=0.03) and global circumferential diastolic SR (p=0.015) measured by STE, although they had LV EF and FS within normal range. This finding suggests that pre-HSCT exposure to anthracyclines might have played a major role in the development of cardiac dysfunction after HSCT in our patients. Our patients received relatively higher cumulative anthracycline doses (median, 293 mg/m2; range, 30-820 mg/m2) compared to those (median, 150 mg/m2; range, 120-195 mg/m2) in children reported by Daly et al.15) This heterogeneity of anthracycline cardiotoxicity might be partially explained by the presence of genetic polymorphisms that might have altered the metabolism of anthracyclines, the myocardial response to the drug, as well as other factors thought to play a role in susceptibility to de novo disease.5)
Post-HSCT medications (such as immunosuppressive agents and corticosteroids) and complications (such as acute GVHD or renal injury) may increase the risk of late-occurring CV complications.5) In this study, 16 patients had acute GVHD needing corticosteroid treatment. They also had significantly decreased LV global longitudinal diastolic SR compared to patients without acute GVHD (p<0.048). LV global longitudinal diastolic SR, an earlier marker of LV diastolic dysfunction, showed negative correlations with BMI (r=-0.503, p=0.001) in acute GVHD patients. These findings suggest that acute GVHD itself or corticosteroids treatment might have affected myocardial diastolic function, although further study is needed to clarify this. Sex and diagnosis (AML vs. ALL) was not associated with LV dysfunction in this study.
Given that cardiovascular complications are the leading causes of late mortality and morbidity in childhood cancer survivors after HSCT, it is important to monitor cardiac function closely with an adequate diagnostic modality to detect myocardial dysfunction earlier in these population. Echocardiography remains the main diagnostic modality. LV EF or FS derived by M-mode imaging is frequently incorporated into follow up protocols of pediatric oncology to monitor LV systolic function, although it has limitations such as geometric assumption and variable reproducibility.16)17) 2D STE is a novel imaging technique to quantify complex cardiac motion with the advantage of angle independency that can measure global and regional ventricular deformation in three dimensions: longitudinal, radial, and circumferential directions.29) 2D STE has been found clinically useful in the assessment of cardiac systolic and diastolic function as well as providing new insights in deciphering cardiac physiology and mechanics in cardiomyopathies and identifying early subclinical changes in various pathologies, including early detection of cardiotoxicity in patients receiving chemotherapy for cancer.18)29) In a study using STE, Cheung et al.18) demonstrated impaired LV myocardial deformation and mechanical dyssynchrony in children after anthracycline therapy despite having normal LV FS. They suggested that the new echocardiographic techniques may unmask the evidence of LV systolic dysfunction in patients who may otherwise be regarded as having normal LV systolic function based on isolated M-mode findings.18) In our study, there was no significant difference in M-mode derived LV EF and FS between patients and controls, with all patients having LV EF and FS within normal limits. However, patients had significantly reduced LV global longitudinal and circumferential myocardial strain and SR compared to normal controls on STE. Since myocardial systolic strain and SR are strong indices of ventricular contractility, it is worth noting that children in this study might experience cardiac dysfunction early after HSCT, although they had normal LV EF and subclinical condition.18)29) The 2D STE is a useful tool to detect early myocardial dysfunction. Therefore, 2D STE may be considered as a routine imaging modality to assess cardiac function in this population. To elucidate the impact of this subclinical early cardiac dysfunction on long term cardiovascular consequences and to provide evidence as to whether this approach would alter long-term outcome in children after HSCT, further longitudinal studies are needed.
There are several limitations in this study. First, since survivors at least more than 6 months after HSCT were included in this study cohort, patients who suffered from acute myocardial dysfunction by high dose of CY and died of it during engraftment period after HSCT might have been excluded from the study. Therefore, immediate and acute impact of preconditioning treatment on cardiac function could not be evaluated in this study. Second, this is a relatively short-term follow up study after HSCT. Therefore, we do not know whether the cardiac functional parameters will change, improve, or deteriorate over time. Longitudinal follow up study is needed to verify prognostic implication of findings in this study. Third, because of the retrospective nature of this study, some possibly important echocardiographic parameters such as TDI velocities of tricuspid valve annulus or the lateral mitral annulus could not be evaluated. However, we focused on the evaluation of LV longitudinal and circumferential myocardial deformation using 2D STE because there has been no study on this issue in children with acute leukemia after HSCT. Nonetheless, we could obtain important information from this study.
In conclusion, early subclinical myocardial systolic and diastolic dysfunction is evident in children after HSCT for acute leukemia. Novel echocardiograpy imaging techniques such as TDI and STE are sensitive and useful for detecting early change in cardiac function in childhood cancer survivors. Although multiple factor may influence cardiac function, pre-HSCT anthracycline exposure was the main risk factor for cardiac dysfunction with minimal effect from pre-HSCT conditioning regimens or complications after HSCT. Careful longitudinal clinical assessment and serial quantitative echocardiography using new imaging techniques to evaluate systolic and diastolic function should be considered for all patients who had HSCT.
Figures and Tables
Fig. 1
Global left ventricular strain (S), strain rate (SR), and diastolic strain rate (DSR) in patients with antracycline cumulative dose <400 mg/m2 (black bar) compared to those in patients with anthracycline cumulative dose >400 mg/m2 (white bar).

Table 1
Demographic and HSCT characteristics of the study cohort
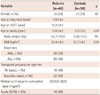
Table 2
Echocardiographic characteristics of patients and controls

*Comparison between pre-HSCT and post-HSCT parameters in patients, †Comparison between post-HSCT parameters in patients and those in controls. HSCT: hematopoietic stem cell transplantation, LV EF: left ventricular ejection fraction, LV FS: left ventricular fractional shortening, LVDD: left ventricular end-diastolic dimension, LVDS: left ventricular end-systolic dimension, Vcfc: rate-corrected velocity of circumferential fiber shortening, DT: deceleration time, MPI: myocardial performance index, SR: strain rate, NS: not significant
References
1. Ballen KK, King RJ, Chitphakdithai P, et al. The national marrow donor program 20 years of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008; 14:9 Suppl. 2–7.
2. Pui CH, Mullighan CG, Evans WE, Relling MV. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood. 2012; 120:1165–1174.
3. Reulen RC, Winter DL, Frobisher C, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010; 304:172–179.
4. Mertens AC. Cause of mortality in 5-year survivors of childhood cancer. Pediatr Blood Cancer. 2007; 48:723–726.
5. Nieder ML, McDonald GB, Kida A, et al. National Cancer Institute-National Heart, Lung and Blood Institute/pediatric Blood and Marrow Transplant Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: longterm organ damage and dysfunction. Biol Blood Marrow Transplant. 2011; 17:1573–1584.
6. Cardous-Ubbink MC, Heinen RC, Langeveld NE, et al. Long-term causespecific mortality among five-year survivors of childhood cancer. Pediatr Blood Cancer. 2004; 42:563–573.
7. MacArthur AC, Spinelli JJ, Rogers PC, Goddard KJ, Abanto ZU, McBride ML. Mortality among 5-year survivors of cancer diagnosed during childhood or adolescence in British Columbia, Canada. Pediatr Blood Cancer. 2007; 48:460–467.
8. Kremer LC, van Dalen EC, Offringa M, Voûte PA. Frequency and risk factors of anthracycline-induced clinical heart failure in children: a systematic review. Ann Oncol. 2002; 13:503–512.
9. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003; 97:2869–2879.
10. Lähteenmäki PM, Chakrabarti S, Cornish JM, Oakhill AH. Outcome of single fraction total body irradiation-conditioned stem cell transplantation in younger children with malignant disease--comparison with a busulphan-cyclophosphamide regimen. Acta Oncol. 2004; 43:196–203.
11. Murdych T, Weisdorf DJ. Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977-1997. Bone Marrow Transplant. 2001; 28:283–287.
12. Braverman AC, Antin JH, Plappert MT, Cook EF, Lee RT. Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol. 1991; 9:1215–1223.
13. Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood. 2011; 118:1413–1420.
14. Armenian SH, Sun CL, Mills G, et al. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010; 16:1138–1144.
15. Daly KP, Colan SD, Blume ED, et al. Changes in echocardiographic measures of systolic and diastolic function in children 1 year after hematopoietic SCT. Bone Marrow Transplant. 2011; 46:1532–1539.
16. Children's Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers. Version 4.0. Arcadia, CA: Children's Oncology Group;2013. Available from: www.survivorshipguidelines.org.
17. Lipshultz SE, Easley KA, Orav EJ, et al. Reliability of multicenter pediatric echocardiographic measurements of left ventricular structure and function: the prospective P(2)C(2) HIV study. Circulation. 2001; 104:310–316.
18. Cheung YF, Hong WJ, Chan GC, Wong SJ, Ha SY. Left ventricular myocardial deformation and mechanical dyssynchrony in children with normal ventricular shortening fraction after anthracycline therapy. Heart. 2010; 96:1137–1141.
19. Alehan D, Sahin M, Varan A, Yıldırım I, Küpeli S, Büyükpamukçu M. Tissue Doppler evaluation of systolic and diastolic cardiac functions in long-term survivors of Hodgkin lymphoma. Pediatr Blood Cancer. 2012; 58:250–255.
20. Baysal T, Koksal Y, Oran B, Sen M, Unal E, Cimen D. Cardiac functions evaluated with tissue Doppler imaging in childhood cancers treated with anthracyclines. Pediatr Hematol Oncol. 2010; 27:13–23.
21. Rathe M, Carlsen NL, Oxhøj H. Late cardiac effects of anthracycline containing therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007; 48:663–667.
22. Shankar SM, Marina N, Hudson MM, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics. 2008; 121:e387–e396.
23. Chow PC, Liang XC, Cheung EW, Lam WW, Cheung YF. New two-dimensional global longitudinal strain and strain rate imaging for assessment of systemic right ventricular function. Heart. 2008; 94:855–859.
24. Mavinkurve-Groothuis AM, Weijers G, Groot-Loonen J, et al. Interobserver, intraobserver and intrapatient reliability scores of myocardial strain imaging with 2-d echocardiography in patients treated with anthracyclines. Ultrasound Med Biol. 2009; 35:697–704.
25. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006; 355:1572–1582.
26. Bu'Lock FA, Mott MG, Oakhill A, Martin RP. Left ventricular diastolic function after anthracycline chemotherapy in childhood: relation with systolic function, symptoms, and pathophysiology. Br Heart J. 1995; 73:340–350.
27. van Dalen EC, van der Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006; 42:3191–3198.
28. Chow EJ, Mueller BA, Baker KS, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011; 155:21–32.
29. Biswas M, Sudhakar S, Nanda NC, et al. Two- and three-dimensional speckle tracking echocardiography: clinical applications and future directions. Echocardiography. 2013; 30:88–105.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download