Abstract
Spontaneous coronary artery dissection (SCAD) is a rare and occasionally life-threatening cause of acute coronary syndrome. Patients may present with clinical scenarios ranging from angina pectoris to cardiogenic shock to sudden cardiac death, and it may be a potentially life-threatening condition if not recognized. However, its etiology, pathophysiology and optimal therapeutic strategies have not been well understood. SCAD is diagnosed on the basis of coronary angiography, but complementary techniques as such intravascular ultrasound (IVUS) and optical coherence tomography should be considered for diagnostic clarification where appropriate. Likewise, the selection of treatment strategy depends upon the clinical manifestation, location and the extent of dissection and amount of ischemic myocardium at risk. Herein, we present the case of a 35-year-old woman who presented with acute myocardial infarction. She was diagnosed by IVUS with spontaneous diffuse dissection of the left anterior descending artery without atheroma, treated with percutaneous coronary stenting, and had a favorable clinical course and was discharged on medical therapy.
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome (ACS) and sudden cardiac death.1) Since this condition was first described by Pretty2) in 1931, it has been reported to have an angiographic incidence of 0.1-1.1%3) with greater prevalence in young females. Patients may present with clinical scenarios ranging from angina pectoris to cardiogenic shock to sudden cardiac death,4) and it may be a potentially life-threatening condition if not recognized. However its etiology, pathogenesis and therapeutic strategies have not as yet been fully defined. We report herein a case of a 35-year-old woman free from any traditional cardiovascular risk factors, who presented with non ST elevation myocardial infarction, was diagnosed with SCAD and treated with percutaneous coronary intervention (PCI).
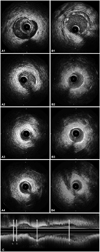
A 35-year-old woman was transferred to the emergency room with acute onset chest pain radiating to the neck. She was a teacher of mathematics and her symptoms initially developed during a lecture. She had no past medical history of collagen tissue disease or blunt trauma to the chest, was not on any medication and had a family history of hypercholesterolemia. She reported no autoimmune disease, was not pregnant or in the early post-partum period, nor did she take an oral contraceptive pill or report any history of drug abuse. She was also not menstruating at time of admission. Risk factors for coronary artery disease were not found. At presentation, her blood pressure was 116/70 mm Hg and her heart rate was 75 beats/min. Her physical examination results were within normal limits without murmurs, rubs or gallops. There were no manifestations of connective tissue disease and the hematologic, renal parameters were normal. The initial electrocardiogram showed nonspecific T wave inversion in leads III, aVF (Fig. 1) and the initial chest X-ray was normal without cardiomegaly or pulmonary edema. Cardiac enzymes were elevated with initial serum troponin T of 0.07 mcg/L (normal <0.014 mcg/L), creatinine kinase (CK) of 138 U/L (normal <215 U/L) and CK-MB of 8.49 mcg/L (normal <3.77 mcg/L). The total cholesterol, triglyceride, high density lipoprotein-cholesterol and low density lipoprotein-cholesterol results were 162 mg/dL (139-230 mg/dL), 33 mg/dL (48-177 mg/dL), 64 mg/dL (40-81 mg/dL) and 106 mg/dL (64-160 mg/dL), respectively. Transthoracic echocardiography demonstrated normal left ventricular systolic function, with an ejection fraction of 73% and no regional wall motion abnormalities. We performed coronary angiography under suspicion of myocardial infarction. The right coronary artery (RCA) and left circumflex artery images were normal, but there was diffuse stenotic lesion of the mid left anterior descending artery (LAD) with up to 80% luminal narrowing. The lesion slightly improved after intracoronary injection of nitroglycerine (Fig. 2). To evaluate the etiology of the stenotic lesion, in terms of spasm, atherosclerosis or other cause, intravascular ultrasound (IVUS, Boston scientific Co., CA, USA) was performed. IVUS showed an isolated dissection flap composed of all of the intima and media, including the internal elastic lamina and external elastic lamina, compressed by hematoma in the false lumen at the bifurcation edge of the mid LAD and diagonal branch. The dissection entry tear site to the false lumen was not detected on angiogram. Furthermore, this site was not clearly detected even on IVUS, and there was no communication between the false and true lumen (Fig. 3A1-A4). Atherosclerotic change was not detected in this vessel (LAD) on the IVUS. 3.0×28 and 2.5×23 mm sized two Xience Prime stents (Abbott Vascular, Santa Clara, CA, USA) were deployed over the dissection from the distal portion of the mid LAD for preventing any subsequent propagation of the dissection. Subsequent angiography revealed no residual stenosis without secondary dissection and restoration of Thrombolysis in Myocardial Infarction (TIMI) grade 3 flow (Fig. 3B). Subsequent IVUS demonstrated a fully covered dissection lesion (Fig. 3B1-B4).
The patient had a favorable clinical course and was discharged on aspirin, clopidogrel, beta blocker and statin. She remained asymptomatic without any complications at six month follow-up.
Spontaneous coronary artery dissection is characterized by the presence of hematoma or thrombus in the false lumen, usually in the outer third of the vessel media, and it can occur primarily or secondarily.5)6) SCAD results in separation of the layers of the coronary arterial wall leading to intramural hematoma into the false lumen, which compression the true lumen and restricts coronary blood flow, leading to coronary ischemia or infarction.6) The first case of angiographic diagnosis was reported almost 50 years after the first description by Pretty2) and PCI and surgical bypass were first reported in the mid-1980s.7)
The mechanism underlying coronary dissection in atheromatous lesion is explained by the increased density of the vasa vasorum and elevated shear stress leading to rupture of the intima, thereby forming a subintimal hematoma and finally resulting in a dissection.8)9) However, the pathophysiology of SCAD is not well established. The probable mechanisms associated with SCAD have been reported so that patients can be separated into three different pathophysiologic groups according to their condition: 1) the non-atherosclerotic, peripartum period group; 2) the atherosclerotic group and 3) the idiopathic or heterogeneous group in which the following other conditions are present: connective tissue disorders (Marfan syndrome, Ehlers-Danlos syndrome, polyarteritis nodosa and systemic lupus erythematous); medication (such as an oral contraceptive, 5 FU, fenfluramine, cyclosporine); cocaine use; hypertension and cystic medial necrosis; variant angina and physical exercise.10) The most common conditions related to SCAD are coronary atherosclerosis and conditions associated with the peripartum period. In the atherosclerotic group, atherosclerotic plaque inflammation and rupture may induce disruption of the intimal-medial junction, leading to an intimal flap and subsequent intramural hematoma formation.10)11) In contrast, peripartum dissection is not related with atherosclerotic plaque. It has been reported that eosinophils infiltrate the uterus and serum collagenase levels increase during labor and the peripartum period. Eosinophilic infiltration in the coronary artery adventitia breaks down the medial-adventitial layer, leading to coronary artery dissection.3)9)12) In the case presented herein, the patient was most likely in the idiopathic group because she did not have atherosclerotic risk factors and was not in the peripartum period, but may have had a strenuous status at time of onset due to her profession. The IVUS findings indicated that the dissection in this case developed in the subadventitia, and there was no communication between the true and false lumen. We could not find any evidence of connective tissue disorder or history of drug abuse. The patient was also not in the menstruation period.
Spontaneous coronary artery dissection is diagnosed on the basis of coronary angiography in most cases, but it can be underestimated using angiography alone.13) Coronary angiography would simply indicate a radiolucent intimal flap or a delayed clearance of contrast from the false lumen. If there is not an intimal tear, the medial hematoma may then appear as a narrowed vessel using consecutive coronary angiography.14) In this situation, it may be difficult to distinguish atherosclerotic stenosis or spasm from intramural hematoma or dissection. IVUS, as a complementary technique, can identify the exact location of the dissection, the relation between the false and the true lumen and the dissection flap.15) The incidence of SCAD has been reported to be about 0.10% in recent angiographic studies. However, Hering et al.3) reported a much higher prevalence of 1.10% in a prospective series using IVUS. In addition, multidetector computed tomography for coronary angiography and optical coherence tomography (OCT) have also been used to identify the extent and thickness of the hematoma.16)17) In our case, we at first suspected spasm because there was no dissection or false lumen visible when we performed only coronary angiography. We could finally diagnose coronary artery dissection by performing IVUS. Consideration of other causes such as SCAD is very important in young healthy females when it is difficult to explain the pathogenesis of the stenotic lesion and there are no atherosclerotic risk factors, particularly considering the diagnostic limitation of coronary angiography alone. The LAD is the most common site of dissection in female patients, whereas the RCA is often affected in male patients.18)
The optimal treatment for SCAD has not been clearly determined and there is no consensus as to recommendations. The selection of treatment strategy depends upon the clinical manifestation, location and extent of dissection and the amount of ischemic myocardium at risk.1) Options include medical therapy, PCI and coronary artery bypass surgery.19) Conservative medical therapy is a reasonable first option in mid- or distal single vessel dissection with a lumen diameter limitation <50% and TIMI grade 2 or 3 flow in the affected coronary artery.5)9)19) However, some studies have demonstrated that early intervention with either PCI or coronary artery bypass graft (CABG) following the diagnosis of SCAD leads to a better outcome.20) The role of thrombolytics is controversial, associated with the extension of dissection due to increased blood in the vessel wall or hematoma causing compression of the true lumen.9) Second, PCI is the therapy of choice in single vessel disease, especially proximal dissection, in which there are ongoing symptoms and persistent restriction of coronary blood flow (TIMI grade 0 or 1 flow). Given that PCI is associated with several complications relating to the passage of the coronary wire into the false lumen of the dissected vessel or the loss of coronary flow through the propagation of dissection and displacement of stents, resulting in the propagation of hematoma, PCI could be limited to cases in which there are ongoing symptoms of ischemia.5) However, there has been no consensus on the appropriate extent of stent coverage in terms of whether there should be proximal portion coverage only or full coverage of the dissection area, because, as occurred in our case, there is no communication between true and false lumen. As such, further prospective randomized trial is needed to confirm the use of PCI in such cases. Likewise, adjunctive imaging technologies such as IVUS and OCT should be considered to determine the extent of dissection and to provide real-time guidance about intervention. In the present case, we performed PCI for persistent ongoing chest pain and IVUS was used for determine the extent and location of the dissection. Third, CABG is the treatment of choice in multiple vessel disease, especially where there is left main stem involvement with on going ischemia refractory to medical or interventional therapy and when there is hemodynamic instability.9) In our case, the dissection was distributed from a relatively proximal location of the bifurcation site to the distal mid LAD, therefore we performed PCI on the affected coronary artery. We also covered the entire lesion with coronary stents, including the hematoma, so as to prevent propagation to the distal vessel.
In conclusion, SCAD is a very rare but serious cause of ACS. The suspicion of SCAD is very important in the differential diagnosis of chest pain with no risk factors for atherosclerosis, especially in healthy young females. Furthermore, IVUS is an important additional tool for diagnosis and therapeutic planning for SCAD.
Figures and Tables
Fig. 2
Coronary angiogram imaging, showing RAO cranial view of the patient LAD. A: long, smooth narrowed lesion extending from the bifurcation point to mid LAD; the length of the narrowed lesion is marked by arrows. B: after intracoronary nitroglycerine infusion, the luminal narrowing of the mid LAD improved slightly, but not completely. RAO: right anterior oblique, LAD: left anterior descending artery.
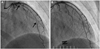
Fig. 3
A: IVUS imaging before (A) and after (B) coronary stenting, and longitudinal imaging before coronary stenting (C). 1: proximal part of dissection. The intima and media are separated from the adventitia and compressed by an echolucent, probably RBC-poor, voluminous huge hematoma. There are two apparent echogenic tram tract like lines, representative of internal and external elastic lamina (A1). This lesion is covered by 3.0×28 mm XiencePrime stent (B1). 2: the most stenotic site on the angiogram. The true lumen is nearly obliterated by echogenic, probably RBC-rich, hematoma on IVUS (A2). This lesion is covered by 2.5×23 mm XiencePrime stent (B2). 3: distal normal segment of the m-LAD showing no atherosclerosis or dissection (A3). Distal stent lands at this normal segment (B3). 4: normal segment of the m-LAD showing just distal part of stent before (A4) and after (B4) coronary stenting. IVUS: intravascular ultrasound, LAD: left anterior descending artery, FL: false lumen, TL: true lumen.
References
1. Almeda FQ, Barkatullah S, Kavinsky CJ. Spontaneous coronary artery dissection. Clin Cardiol. 2004; 27:377–380.
2. Pretty HC. Dissecting aneurysm of coronary artery in a woman aged 42. Br Med J. 1931; 1:667–669.
3. Hering D, Piper C, Hohmann C, Schultheiss HP, Horstkotte D. [Prospective study of the incidence, pathogenesis and therapy of spontaneous, by coronary angiography diagnosed coronary artery dissection]. Z Kardiol. 1998; 87:961–970.
4. Desai S, Sheppard MN. Sudden cardiac death: look closely at the coronaries for spontaneous dissection which can be missed. A study of 9 cases. Am J Forensic Med Pathol. 2012; 33:26–29.
5. Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012; 126:579–588.
6. Adlam D, Cuculi F, Lim C, Banning A. Management of spontaneous coronary artery dissection in the primary percutaneous coronary intervention era. J Invasive Cardiol. 2010; 22:549–553.
7. Gonzalez JI, Hill JA, Conti CR. Spontaneous coronary artery dissection treated with percutaneous transluminal angioplasty. Am J Cardiol. 1989; 63:885–886.
8. Maehara A, Mintz GS, Castagna MT, et al. Intravascular ultrasound assessment of spontaneous coronary artery dissection. Am J Cardiol. 2002; 89:466–468.
9. Paraskevaidis S, Theofilogiannakos EK, Chatzizisis YS, et al. Spontaneous dissection of right coronary artery manifested with acute myocardial infarction. Open Cardiovasc Med J. 2010; 4:178–180.
10. Almafragi A, Convens C, Heuvel PV. Spontaneous healing of spontaneous coronary artery dissection. Cardiol J. 2010; 17:92–95.
11. Borczuk AC, van Hoeven KH, Factor SM. Review and hypothesis: the eosinophil and peripartum heart disease (myocarditis and coronary artery dissection)--coincidence or pathogenetic significance? Cardiovasc Res. 1997; 33:527–532.
12. Robinowitz M, Virmani R, McAllister HA JrU. Spontaneous coronary artery dissection and eosinophilic inflammation: a cause and effect relationship? Am J Med. 1982; 72:923–928.
13. Jorgensen MB, Aharonian V, Mansukhani P, Mahrer PR. Spontaneous coronary dissection: a cluster of cases with this rare finding. Am Heart J. 1994; 127:1382–1387.
14. Arnold JR, West NE, van Gaal WJ, Karamitsos TD, Banning AP. The role of intravascular ultrasound in the management of spontaneous coronary artery dissection. Cardiovasc Ultrasound. 2008; 6:24.
15. Lerakis S, Manoukian S, Martin RP. Transesophageal echo detection of postpartum coronary artery dissection. J Am Soc Echocardiogr. 2001; 14:1132–1133.
16. Regar E, Ligthart J, Bruining N, van Soest G. The diagnostic value of intracoronary optical coherence tomography. Herz. 2011; 36:417–429.
17. Manghat NE, Morgan-Hughes GJ, Roobottom CA. Spontaneous coronary artery dissection: appearance and follow-up on multi-detector row CT coronary angiography. Clin Radiol. 2005; 60:1120–1125.
18. Mortensen KH, Thuesen L, Kristensen IB, Christiansen EH. Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter Cardiovasc Interv. 2009; 74:710–717.
19. Vanzetto G, Berger-Coz E, Barone-Rochette G, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg. 2009; 35:250–254.
20. Shamloo BK, Chintala RS, Nasur A, et al. Spontaneous coronary artery dissection: aggressive vs. conservative therapy. J Invasive Cardiol. 2010; 22:222–228.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download