Abstract
Background and Objectives
Materials and Methods
Results
Acknowledgments
References
Supplementary Material
Fig. 1
Geographic distribution of PCI facilities participating in the K-PCI registry. Ninety-two hospitals submitted data for 44967 PCI cases. High-volume facilities were concentrated in Seoul and metropolitan areas. PCI: percutaneous coronary intervention, K-PCI: Korean percutaneous coronary intervention.
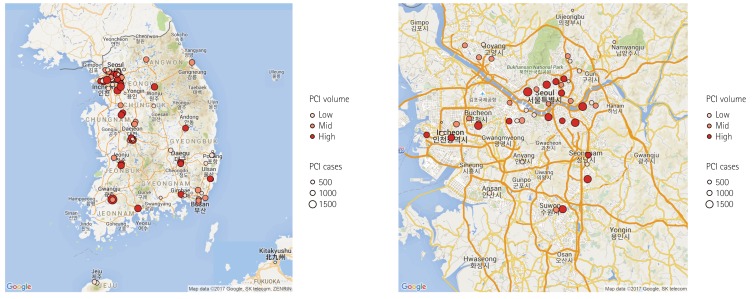
Fig. 2
Distribution of facilities with different PCI volume. Sixty-two percent of the included hospitals performed 500 or fewer PCI procedures and 11% performed more than 1000 PCIs during the collection period. PCI: percutaneous coronary intervention.
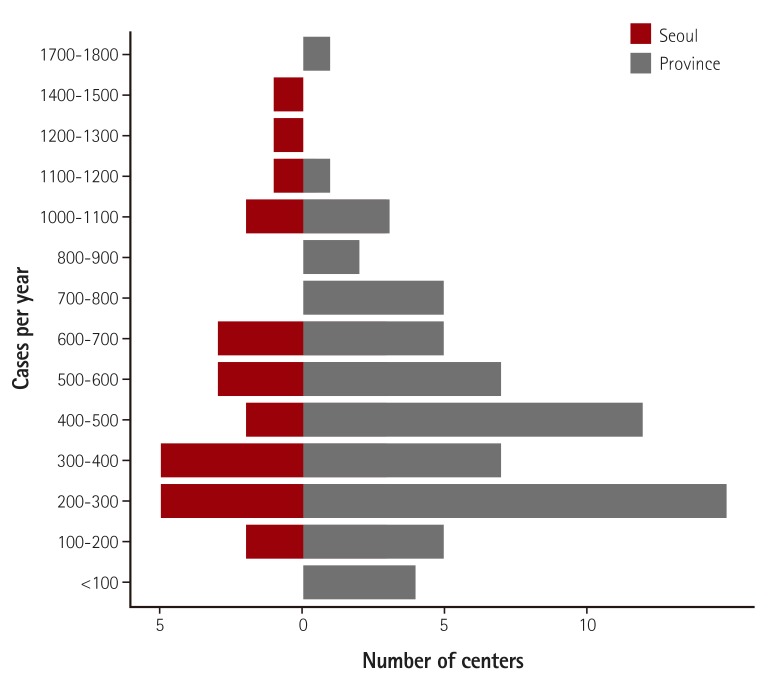
Fig. 3
Age and gender distribution. Median age was 66 years and the proportion of male patients was 70.3%.
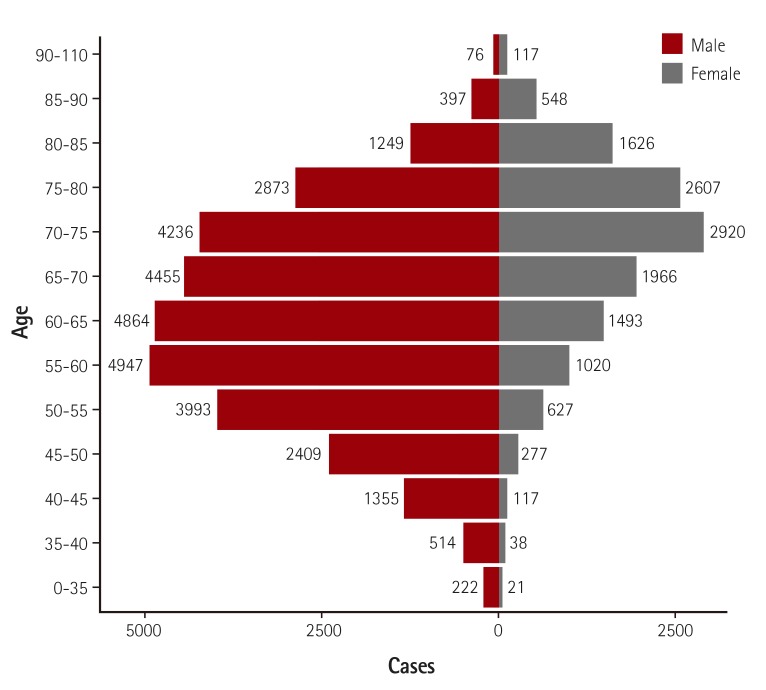
Fig. 4
Gender-specific clinical indications for PCI. The width of the bars in the histogram indicates the number of patients. NSTEMI: non-ST-elevation myocardial infarction, STEMI: ST-elevation myocardial infarction, PCI: percutaneous coronary intervention.
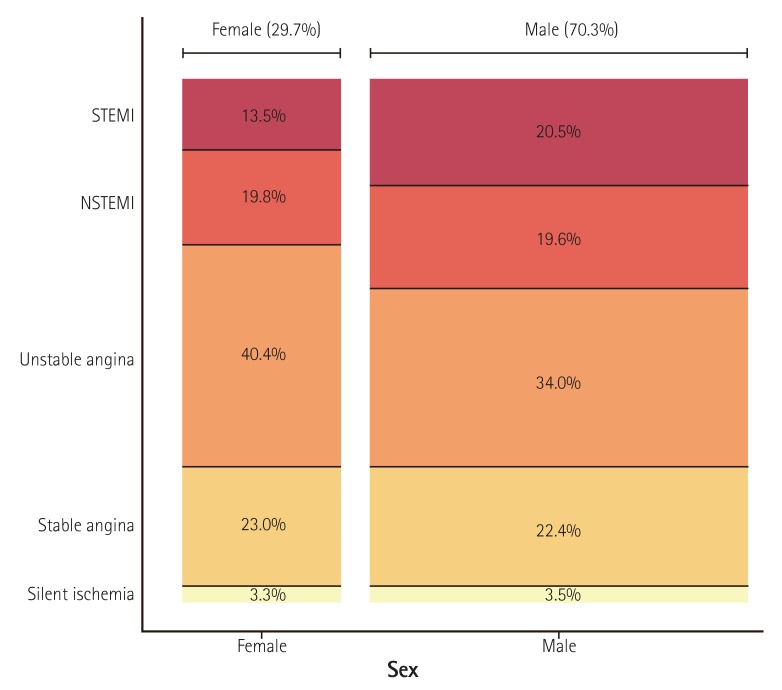
Fig. 5
Age-specific clinical indications for PCI. The width of the bars in the histogram indicates the number of patients. NSTEMI: non-ST-elevation myocardial infarction, STEMI: ST-elevation myocardial infarction, PCI: percutaneous coronary intervention.
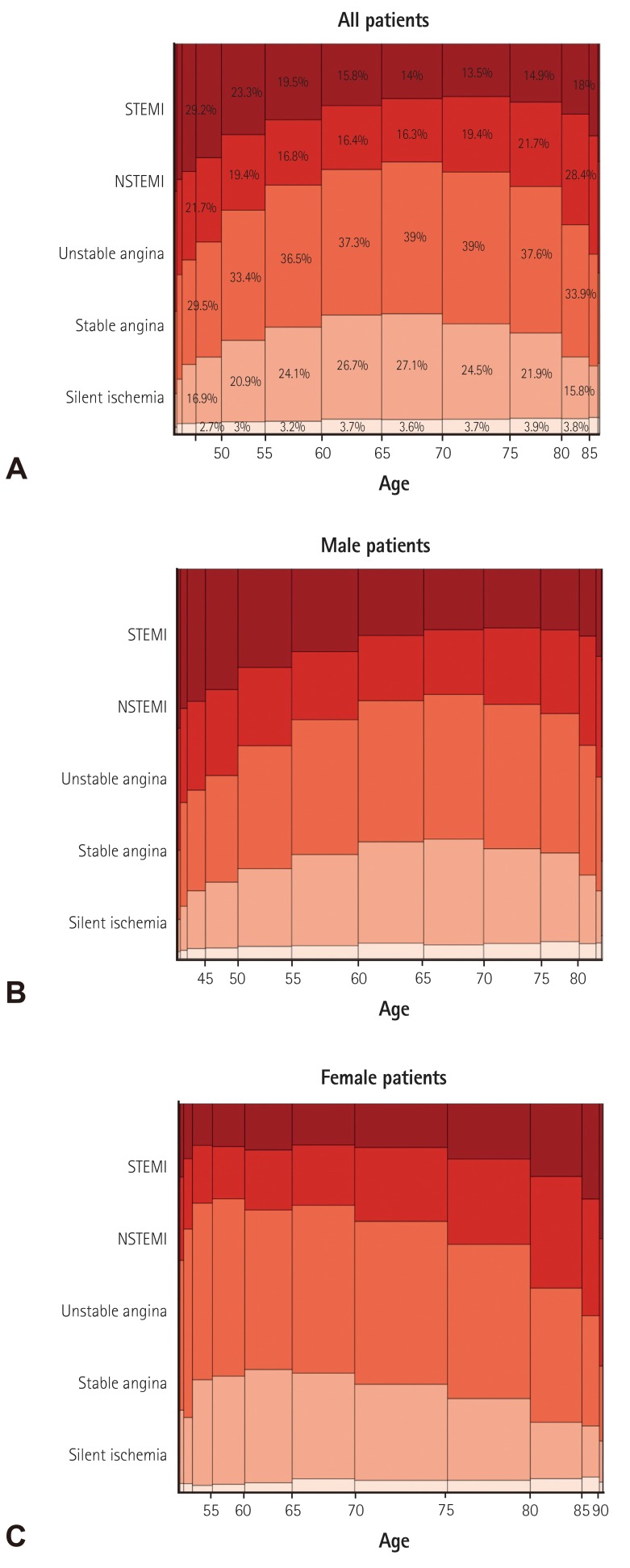
Fig. 6
Temporal distribution of PCI procedures and in-hospital events. (A) Monthly distribution of PCI procedures and in-hospital cardiac events. (B) Weekly distribution of PCI procedures and in-hospital cardiac events. STEMI: ST-elevation myocardial infarction, NSTEMI: non-ST-elevation myocardial infarction, PCI: percutaneous coronary intervention, MACE: major adverse cardiac events.
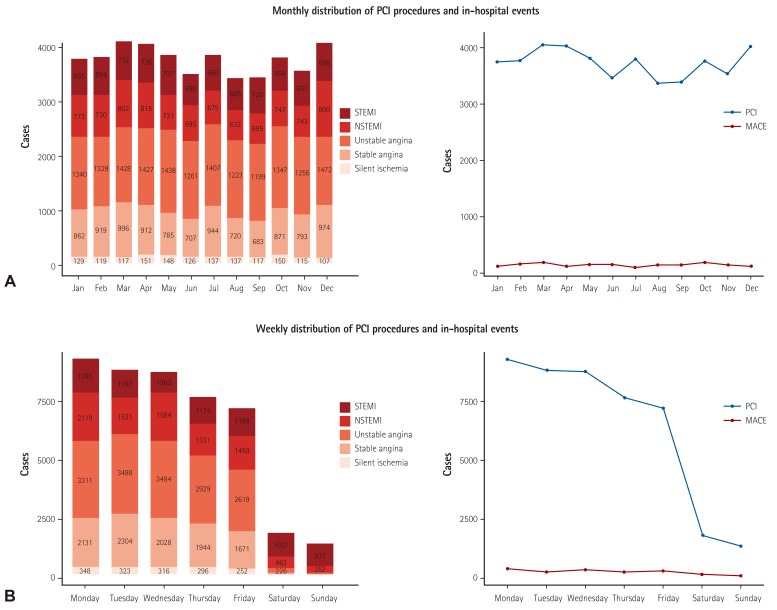
Table 1
Baseline patient and clinical characteristics
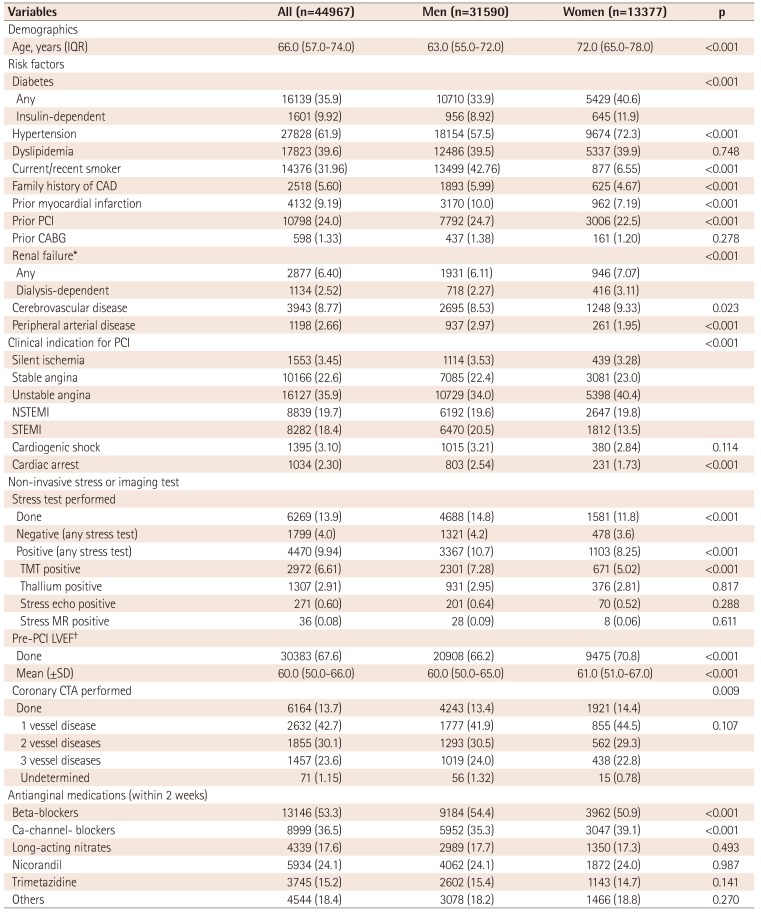
Values are presented as number (%) if not otherwise specified. *GFR ≤60 mL/min/1.73 m2, †can be assessed via invasive (i.e. left ventriculography) or non-invasive (i.e. echocardiography, magnetic resonance, computed tomography, or nuclear) testing. IQR: interquartile range, CAD: coronary artery disease, PCI: percutaneous coronary intervention, CABG: coronary artery bypass graft, NSTEMI: non-ST-elevation myocardial infarction, STEMI: ST-elevation myocardial infarction, TMT: treadmill test, MR: magnetic resonance, LVEF: left ventricular ejection fraction, SD: standard deviation, CTA: computed tomography angiography
Table 2
Baseline angiographic and procedural characteristics
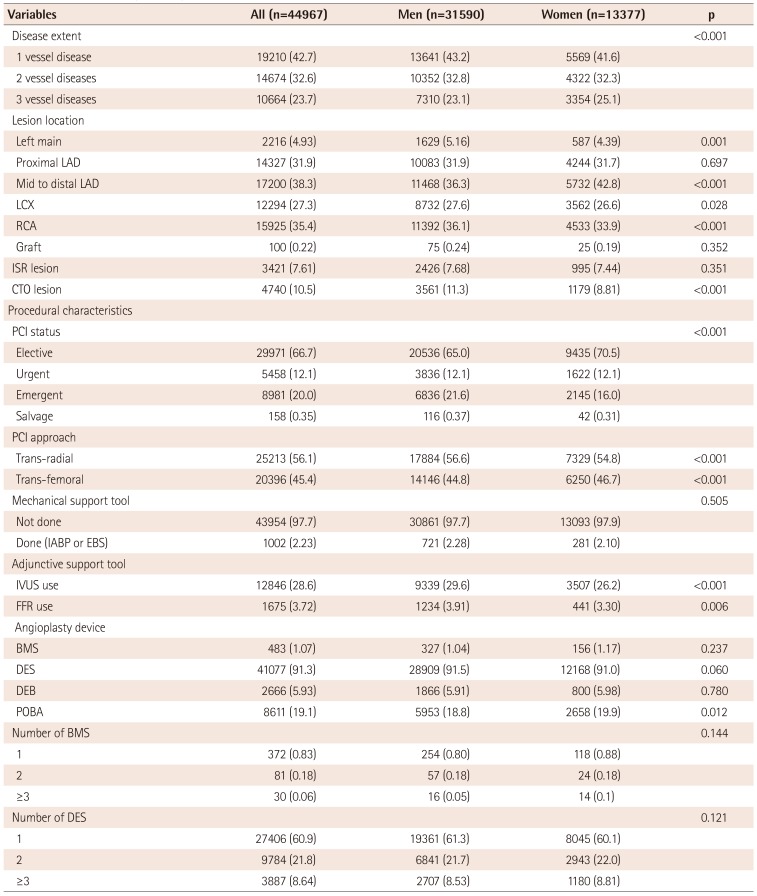
Values are presented as number (%) and percent if not otherwise specified. LAD: left anterior descending, LCX: left circumflex, RCA: right coronary artery, ISR: in-stent restenosis, CTO: chronic total occlusion, PCI: percutaneous coronary intervention, IABP: intra-aortic balloon pump, EBS: emergency bypass system, IVUS: intravascular ultrasound, FFR: fractional flow reserve, BMS: bare-metal stents, DES: drug-eluting stents, DEB: drug-eluting balloon, POBA: plain old balloon angioplasty
Table 3
In-hospital outcomes
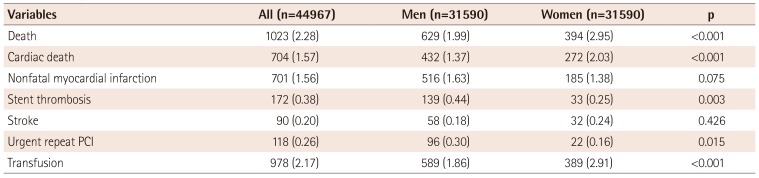
Table 4
Baseline patient and clinical characteristics by PCI case-volume
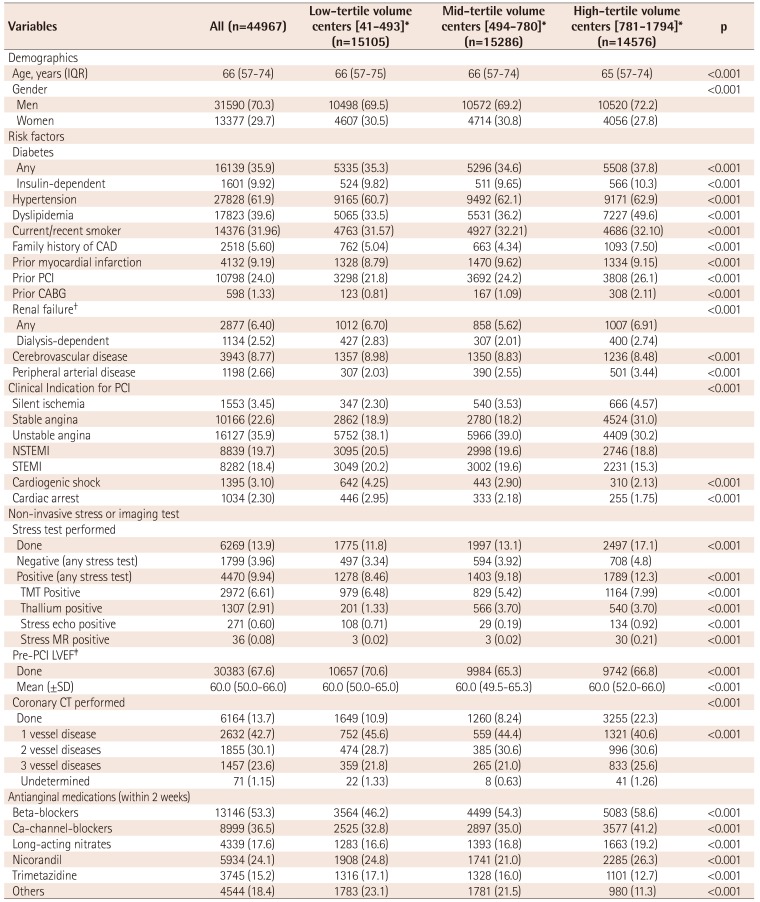
Values are presented as number and percent if not otherwise specified. *Centers were divided into three tertiles based on PCI case-volume (1st tertile: 41-493; 2nd tertile: 494-780; 3rd tertile: 781-1794), †GFR ≤60 mL/min/1.73 m2, ‡can be assessed via invasive (i.e. left ventriculography) or non-invasive (i.e. echocardiography, magnetic resonance, computed tomography, or nuclear) testing. IQR: inter-quartile range, CAD: coronary artery disease, PCI: percutaneous coronary intervention, CABG: coronary artery bypass graft, NSTEMI: non-ST-elevation myocardial infarction, STEMI: ST-elevation myocardial infarction, TMT: treadmill test, MR: magnetic resonance LVEF: left ventricular ejection fraction, VD: vessel disease, SD: standard deviation
Table 5
Baseline angiographic and procedural characteristics by PCI case-volume
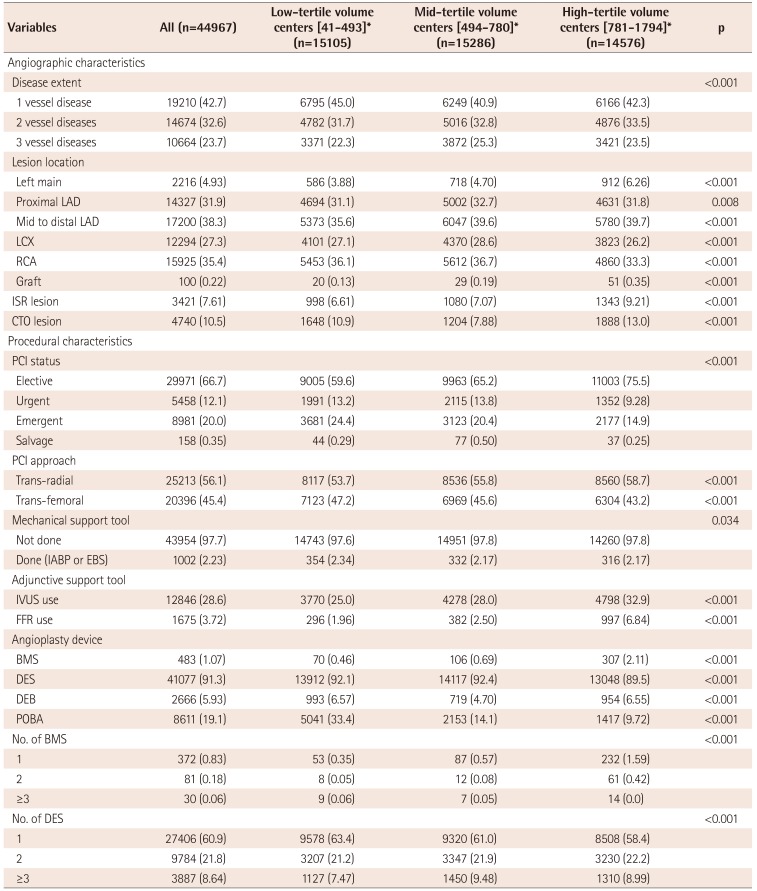
Values are presented as number (%) if not otherwise specified. *Centers were divided into three tertiles based on PCI case-volume (1st tertile: 41-493; 2nd tertile: 494-780; 3rd tertile: 781-1794). LAD: left anterior descending, LCX: left circumflex, RCA: right coronary artery, ISR: in-stent restenosis, CTO: chronic total occlusion, PCI: percutaneous coronary intervention, IABP: intra-aortic balloon pump, EBS: emergency bypass system, IVUS: intravascular ultrasound, FFR: fractional flow reserve, BMS: bare-metal stents, DES: drug-eluting stents, DEB: drug-eluting balloon, POBA: plain old balloon angioplasty
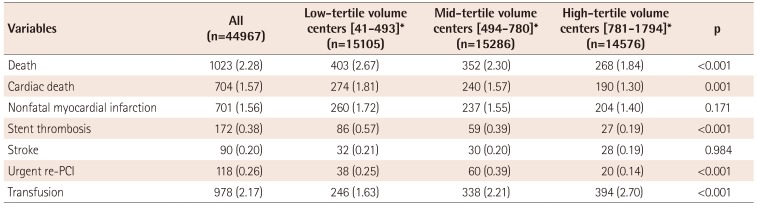
Table 6
In-hospital outcomes by PCI case-volume




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download