Abstract
Background and Objectives
Carotid intima-media thickness (IMT) is associated with chronic inflammation, and C-reactive protein (CRP) level is elevated in patients with atrial fibrillation (AF). We investigated the impacts of CRP and AF on carotid atherosclerosis and ischemic stroke in patients with suspected ischemic cerebrovascular disease.
Subjects and Methods
One-hundred forty patients (78 males) with suspected ischemic cerebrovascular disease underwent carotid ultrasonography. The mean common carotid artery IMT, mean internal carotid artery (ICA) IMT, and plaque score were measured. Patients were divided into four groups according to the presence of AF and elevated CRP level {n=46 for AF(-)CRP(-), n=38 for AF(-)CRP(+), n=43 for AF(+)CRP(-), and n=13 for AF(+)CRP(+)}.
Results
Common carotid artery IMT was significantly higher in the AF(-)CRP(+) (0.98±0.51 mm) and AF(+)CRP(+) (0.96±0.27 mm) groups compared to the AF(-)CRP(-) (0.80±0.32 mm) and AF(+)CRP(-) (0.77±0.19 mm) groups (p=0.027). Although there was no significant difference in mean ICA IMT among the groups, plaque score was the highest in the AF(+)CRP(+) (4.18±3.84 mm) group, followed by AF(-)CRP(+) (3.87±2.78 mm), AF(+)CRP(-) (1.34±2.61 mm), and AF(-)CRP(-) (1.17±2.02 mm) (p<0.001). The AF(+)CRP(+) group showed significantly higher incidence of ischemic stroke than the other groups (all p<0.05). Binary logistic regression analysis showed that age {odds ratio (OR)=1.033, p=0.001}, elevated CRP (OR=3.884, p=0.001), and the presence of AF (OR=1.375, p=0.018) were significantly correlated with incidence of ischemic stroke.
Atherosclerosis is not merely a passive accumulation of lipids within artery walls, but a multifactorial, multistep inflammatory disease that involves chronic inflammation at every stage, from initiation to plaque rupture.1)2) Various cytokines, growth factors, and inflammatory cells are abundant in atheromatous plaques.3)4) C-reactive protein (CRP), interleukin-6, and homocysteine are inflammation-sensitive proteins that have been studied in relation to cardiovascular disease risk.5) Among these, CRP is the most sensitive as a cardiovascular risk factor using Framingham risk scoring and is also easy and cost-effective to measure.6)7) CRP activity is positively correlated with plaque instability as well as intima to media wall thickness of coronary arteries and the common carotid artery (CCA).8-10) Carotid artery intima media thickening (IMT) is a risk factor for myocardial infarction and stroke in older adults.11) Higher CRP is also associated with higher IMT,9)10) although this has not been confirmed in all studies.12)13) It is unclear whether CRP plays an independent role in the carotid atherosclerosis of patients with atrial fibrillation (AF).
Atrial fibrillation is one of the most prevalent and vexing cardiovascular conditions. Acute or chronic hemodynamic, metabolic, or inflammatory stressors may lead to structural and ionic remodeling of the atria, which may promote AF progression and persistence. Recent studies have suggested that inflammation and oxidative stress play a role in the pathogenesis of AF.14)15) There is increased incidence of AF after cardiac surgery,16) and inflammatory cell infiltration, myocardial necrosis, and fibrosis are present in atrial biopsies of patients with lone AF refractory to antiarrhythmic drug therapy.17) There were also elevated CRP levels in patients with AF in a previous prospective randomized study.18)
We hypothesize here that, since chronic inflammation contributes to both AF and atherosclerosis, CRP has an independent additive role in carotid atherosclerosis in patients with AF. Thus, we hypothesized that, if patients with AF have elevated CRP level, carotid IMT or plaque burden would increase more than in patients with elevated CRP level without AF or in patients with AF and normal CRP level. We investigated the impacts of CRP and AF on carotid atherosclerosis and ischemic stroke in patients with suspected ischemic cerebrovascular disease.
One-hundred forty patients with suspected ischemic cerebrovascular disease who underwent carotid ultrasonography (US) and electrocardiography (ECG) and who had CRP level measured were consecutively enrolled in a retrospective observational single-center cohort-study at Kosin University Gospel Hospital. Ischemic cerebrovascular diseases included transient ischemic attack (TIA) and stroke. TIA was defined when cerebral ischemia caused by reduced cerebral blood flow was restored quickly and neurological signs and symptoms lasted less than 24 hours. Stroke was defined when the cessation of cerebral blood flow lasted for more than a few minutes and resulted in cerebral infarction and neurological signs and symptoms lasting more than 24 hours. Patients with chronic congestive heart failure, surgery within 60 days, infection, rheumatologic disorder, immunological disorder, chronic renal insufficiency, or acute coronary syndrome within 30 days before CRP collection were excluded from this study. US quantification of carotid artery IMT was obtained for all patients. Classic cardiovascular risk factors were considered in this study, in addition to the epidemiologic variables of age and sex. Old age was defined as >65 years; hypertension was defined as systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or previous diagnosis; diabetes mellitus was defined as fasting glucose >120 mg/dL, HbA1c >6.5, or previous diagnosis; dyslipidemia was defined as total cholesterol >220 mg/dL, triglyceride >200 mg/dL, or previous diagnosis. The Institutional Review Board of Kosin University School of Medicine approved this study, and all patients gave written informed consent before participation.
Venous blood was drawn the morning after an overnight fast on the first hospitalized day. Complete blood cell counts, serum electrolytes, and thyroid function tests for all patients were within the normal range of standardized values. The following parameters were obtained with standard techniques on the examination day: total cholesterol, low density lipoprotein-cholesterol, high density lipoprotein-cholesterol, triglycerides, high sensitivity C-reactive protein (hs-CRP), and fibrinogen. hs-CRP was measured using fully automated turbid immunometry (Advia 1800, Siemens, Munich, Germany), and a concentration ≥2.0 mg/L was defined as elevated.19) Height and weight were measured, and body mass index (kg/m2) was calculated. Participants rested for at least 10 minutes in the supine position prior to carotid US examination. Normal sinus rhythm with a rate of 60-100 beats/min was required on resting ECG prior to examination.
For all participants, two expert examiners who were blinded to patient medical histories performed the extracranial carotid artery US with IMT measurements and carried out analysis for plaque presence. US scans utilized iE33 (Philips Medical Systems, Best, The Netherlands) equipped with a 7- to 12-MHz linear-array scanner. All participants were examined in the supine position with their necks extended and chins facing the contralateral side. Carotid arteries were examined bilaterally in longitudinal and transversal planes. Two observers who were blinded to participant demographic data and cardiovascular risk measured the combined thickness of the intima and media of both common carotid arteries (CCA). After placing a region of interest in the far wall of the CCA, mean IMT was estimated in a region free of atherosclerotic plaques using an automatic tracking system. Mean IMT was computed from 80 to 120 measurements over a 10-mm span ending 5-mm proximal to the transition between the CCA and bulb regions. The maximum IMT of the CCA and internal carotid artery (ICA) was defined as the mean of multiple measures of the maximum IMT of the near and far wall on both left and right sides. A composite measure that combined the maximum CCA IMT and maximum ICA IMT was obtained by averaging these two measurements after standardization {subtraction of the mean and division by the standard deviation (SD) for measurements}. An increased IMT was defined as ≥1.0 mm increase in either or both carotid arteries, and the presence of atherosclerotic plaque was defined as a focal structure that encroached into the arterial lumen by at least 0.5 mm or 50% of the surrounding IMT value or demonstrated a thickness >1.5-mm as measured from the media - adventitial interface to the intima - lumen interface.20) The maximal thickness of each plaque was measured perpendicular to the vascular wall, and plaque score was computed by summing the thickness of all plaques located in bilateral carotid arteries.21) Intra- and inter-operator coefficients of variation were 2.9% and 3.0%, respectively, and intra- and inter-operator intra-class correlations were both 0.96.
Electrocardiographys of all selected patients were reviewed, and patients with a sinus rhythm were placed on 24-48 hour Holter monitoring to monitor for paroxysmal AF. Subjects with 12-lead ECGs demonstrating AF or arrhythmia >30 seconds in duration on Holter monitoring or telemetry recordings were considered to have AF.
Statistical analysis was performed with Statistical Package for the Social Sciences (SPSS) for Windows version 12.0 (SPSS Inc., Chicago, IL, USA). Results are presented as mean±SD or percentage. To analyze the relationship between CRP or presence of AF on carotid atherosclerosis, study patients were divided into four subgroups: absence of AF with normal CRP level, AF(-)CRP(-); absence of AF with elevated CRP level, AF(-)CRP(+); presence of AF with normal CRP, AF(+)CRP(-); and presence of AF with elevated CRP level, AF(+) CRP(+). The differences between categorical variables were determined using the χ2-test. The independent Student's t-test was used to determine the difference in normally distributed data, and the Mann-Whitney U test was used for comparison of median for the non-parametrically distributed variables. The statistical differences among the parametric data of the four groups were analyzed using the one-way analysis of variance test, and the differences between the subgroups were assessed with the post-hoc Tukey test. In the presence of a statistically significant difference, the single post-test comparison was made using the Mann-Whitney U test, and the Bonferroni correction was applied for multiple comparisons. Correlations between variables were made using Pearson correlation tests, and inter- (or intra-) observer variation was tested by Spearman correlation coefficients. Binary logistic regression analysis was used to determine the independent and synergistic impacts of CRP or AF on carotid IMT, plaque and incidence of stroke using selected continuous and discrete risk factor traits as covariates. Statistical significance was set at 0.05.
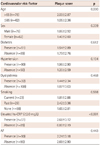
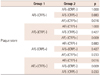
Enrolled patients included those with ischemic stroke (n=96) and TIA (n=44). There were no significant differences in the baseline clinical characteristics of study patients according to the presence of AF, except that patients with AF had enlarged cardiac chamber dimensions and reduced ejection fraction (Table 1). A comparison of the cardiovascular risk factors, carotid atherosclerosis parameters, and incidence of ischemic cerebrovascular disease among subgroups is shown in Table 2. There were no significant differences in the cardiovascular risk factors among the groups. Mean CCA IMT was significantly higher in AF(-)CRP(+) and AF(+)CRP(+) groups compared to AF(-)CRP(-) and AF(+)CRP(-) groups (p<0.05). Although there was no significant difference in mean ICA IMT among groups, presence of plaque, plaque score, and incidence of ischemic stroke were highest in AF(+)CRP(+), followed by AF(-)CRP(+), AF(+)CRP(-), and AF(-)CRP(-) (Table 2). Plaque scores differed significantly according to age and CRP level among various cardiovascular risk factors (Table 3). Analysis by Bonferroni test revealed that only plaque score was significantly different among groups (Table 4).
There was significant positive correlation between the presence of elevated hs-CRP level and the increased carotid IMT (r=0.359, p<0.001), the presence of plaque (r=0.512, p<0.001), as well as incidence of ischemic stroke (r=0.313, p=0.016). To investigate the additive effect of AF and hs-CRP on carotid atherosclerosis and the incidence of ischemic stroke, a multivariate regression test was performed (Table 5). Binary logistic regression analysis showed that age {odds ratio (OR)=1.033, p=0.001}, elevated CRP (OR=3.884, p=0.001), and the presence of AF (OR=1.375, p=0.018) were significantly related to incidence of ischemic stroke. To investigate the synergistic effect of AF and hs-CRP, we included the subgroup phenotypes in another binary logistic regression analysis (Table 5). AF(+)CRP(+) was the strongest combination related to increased carotid IMT (OR=7.222, p<0.001), presence of plaque (OR=6.857, p<0.001), and incidence of ischemic stroke (OR=13.000, p=0.018).
This study showed that elevated CRP concentration was associated with carotid atherosclerosis in patients with AF. Moreover, AF patients with elevated CRP showed the highest incidence of ischemic stroke, implying that elevated CRP and the presence of AF are synergistic contributing factors of carotid atherosclerosis and ischemic stroke. To the best of our knowledge, this is the first study to explore the synergistic associations of CRP and AF with carotid atherosclerosis and incidence of ischemic stroke.
We confirmed here that elevated CRP level is related to increased carotid IMT and plaque.9)10)22) Because atherosclerotic vessel walls are likely sources of measurable systemic inflammation, elevated CRP in part reflects the burden of atherosclerosis. In the present study, the close association of CRP and carotid atherosclerotic plaque score might indicate an association between higher CRP and more active or unstable plaques, which have greater propensity to cause stroke.22)23) Elevated CRP level is also involved in the development and continuation of AF,18)24) which is an important risk factor for stroke25)26) and carotid atherosclerosis.11) CRP also may have prothrombotic effects due to increased tissue factor expression. The association between CRP and thromboembolic risk could be related to an association between CRP and AF.27) CRP level may be useful in determining risk for stroke and thromboembolism as well as the need for anticoagulant therapy in patients with AF.18)
Strengths of the present study include the synergistic effect of elevated CRP and AF on carotid atherosclerosis and incidence of ischemic stroke. AF patients with elevated CRP have a higher plaque burden and increased mean CCA IMT compared to non-AF patients with elevated CRP. However, AF alone did not independently impact carotid IMT or plaque score. The incidence of ischemic stroke was also higher in AF patients with elevated CRP than in patients of other subgroups.
There are many factors that affect carotid IMT, including known cardiovascular risk factors and prescribed medications. Other cardiovascular risk factors that may increase carotid IMT were distributed statistically through the four subgroups, so the possibility of epidemiological selection bias was excluded. The second limitation is the concern regarding the clinical significance of differentiating between TIA and ischemic stroke. TIA is typically thought to resolve before permanent damage, but TIA also may be considered an ischemic penumbra of varied duration, which could proceed to cerebral infarction or reduce to benign oligemia.28) TIA may be a benign or prodromal phenomenon of ischemic stroke. Other limitations include a relatively small number of study patients with heterogeneity of persistent or paroxysmal AF. The findings should be further explored in studies with a large population with carotid disease and stroke risk factors, including other population-based cohorts. Another limitation is that IMT and CRP were determined by a single measure, which could result in imprecision or residual confounding due to measurement error. This would bias the findings toward the null hypothesis to underestimate the actual risk associated with these measures.
We conclude that elevated plasma CRP concentration may be a reliable surrogate marker for predicting carotid atherosclerosis severity in patients with AF, and that CRP concentration may be related to an increased risk of ischemic stroke. AF patients with elevated CRP are likely to have more advanced atherosclerosis and increased chance of cerebrovascular accidents. Anti-inflammatory drugs such as statins and anticoagulation medications might be required for these patients. The findings should be further explored in laboratory, observational, and interventional studies.
Figures and Tables
Table 1
Clinical characteristics and carotid artery parameters in the study population according to the presence of atrial fibrillation

All values are presented as the mean±SD (continuous variables) or number (categorical variables). AF: atrial fibrillation, BMI: body mass index, LDL-C: low density lipoprotein-cholesterol, HDL-C: high density lipoprotein-cholesterol, TG: triglycerides, hs-CRP: high sensitivity C-reactive protein, TSH: thyroid stimulating hormone, LVEDD: left ventricular end diastolic dimension, LVESD: left ventricular end systolic dimension, CCA: common carotid artery, IMT: intima media thickness, ICA: internal carotid artery
Table 2
Comparison of cardiovascular risk factors, parameters of carotid atherosclerosis, and incidence of ischemic cerebrovascular disease among the study groups

References
1. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002; 105:1135–1143.
2. Shah PK. Plaque disruption and thrombosis. Potential role of inflammation and infection. Cardiol Clin. 1999; 17:271–281.
3. Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986; 6:131–138.
4. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999; 340:115–126.
5. Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004; 109:25 Suppl 1. IV6–IV19.
6. Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004; 350:1387–1397.
7. Koenig W, Löwel H, Baumert J, Meisinger C. C-reactive protein modulates risk prediction based on the Framingham Score: implications for future risk assessment: results from a large cohort study in southern Germany. Circulation. 2004; 109:1349–1353.
8. Lombardo A, Biasucci LM, Lanza GA, et al. Inflammation as a possible link between coronary and carotid plaque instability. Circulation. 2004; 109:3158–3163.
9. Winbeck K, Kukla C, Poppert H, Klingelhöfer J, Conrad B, Sander D. Elevated C-reactive protein is associated with an increased intima to media thickness of the common carotid artery. Cerebrovasc Dis. 2002; 13:57–63.
10. Alvarez Garcia B, Ruiz C, Chacon P, Sabin JA, Matas M. High-sensitivity C-reactive protein in high-grade carotid stenosis: risk marker for unstable carotid plaque. J Vasc Surg. 2003; 38:1018–1024.
11. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Cardiovascular Health Study Collaborative Research Group. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999; 340:14–22.
12. Folsom AR, Pankow JS, Tracy RP, et al. Association of C-reactive protein with markers of prevalent atherosclerotic disease. Am J Cardiol. 2001; 88:112–117.
13. Tracy RP, Psaty BM, Macy E, et al. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler Thromb Vasc Biol. 1997; 17:2167–2176.
14. Cai H, Li Z, Goette A, et al. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002; 106:2854–2858.
15. Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003; 108:3006–3010.
16. Bruins P, te Velthuis H, Yazdanbakhsh AP, et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997; 96:3542–3548.
17. Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997; 96:1180–1184.
18. Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001; 104:2886–2891.
19. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008; 359:2195–2207.
20. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012; 34:290–296.
21. Nagai Y, Kitagawa K, Yamagami H, et al. Carotid artery intima-media thickness and plaque score for the risk assessment of stroke subtypes. Ultrasound Med Biol. 2002; 28:1239–1243.
22. Blackburn R, Giral P, Bruckert E, et al. Elevated C-reactive protein constitutes an independent predictor of advanced carotid plaques in dyslipidemic subjects. Arterioscler Thromb Vasc Biol. 2001; 21:1962–1968.
23. Burke AP, Tracy RP, Kolodgie F, et al. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002; 105:2019–2023.
24. Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003; 108:3006–3010.
25. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994; 271:840–844.
26. Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982; 306:1018–1022.
27. Cermak J, Key NS, Bach RR, Balla J, Jacob HS, Vercellotti GM. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993; 82:513–520.
28. Hadjiev DI, Mineva PP. A reappraisal of the definition and pathophysiology of the transient ischemic attack. Med Sci Monit. 2007; 13:RA50–RA53.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download