Abstract
Background and Objectives
The aim of this study was to examine the anti-proliferative and anti-inflammatory effects of a stent coated with abciximab and alpha-lipoic acid (ALA) in a porcine coronary overstretch restenosis model.
Materials and Methods
A total of 10 pigs were randomized into two groups (10 pigs, 10 coronaries in each group) in which the coronary arteries were stented with a dual-coated stent and a bare metal stent (control) by randomization. Stents were deployed with oversizing (stent/artery ratio 1.3 : 1) in the porcine coronary arteries, and histopathology was assessed 28 days after stenting.
Results
There was no significant difference in the injury score between the two groups. In the neointima, the lymphohistiocyte count was significantly lower in dual-coat stent group compared with the control stent group (120±85 cells vs. 159±80 cells, p=0.048). There was no significant difference in the fibrin score between the two groups (0.16±0.34 in the dual-coated stent group vs. 0.25±0.48 in the control stent group, p=0.446). The neointima area was not significantly different between both groups (1.55±0.8 mm2 in dual-coated stent group vs. 1.40±0.86 mm2 in the control stent group, p=0.447).
Conclusion
Although the dual-coated stent with abciximab and ALA showed no significant difference in inhibition of neointimal hyperplasia when compared with the bare metal stent, it was associated with a reduced inflammatory reaction when compared with the control stent in a porcine coronary restenosis model.
The use of coronary stents has increased significantly in recent years;1) however, in-stent restenosis (ISR) after stent implantation is major adverse effect.2-4) The principal mechanism of ISR is thrombosis and neointimal hyperplasia, and it has been reported that there is a strong correlation between the degree of vascular inflammation and neointimal formation.5-9) The pathological process of ISR is characterized by an inflammatory healing process after stretch and damage of the vessel wall.10)
Abciximab (Reopro®) is a potent anti-platelet agent. Systemic use of abciximab has significantly reduced thrombotic complications following complex angioplasty and stenting.11-14) Alpha lipoic acid (ALA) is a potent antioxidant and acts as a cofactor of key mitochondrial enzymes such as pyruvate dehydrogenase and α-ketoglutarate dehydrogenase.15) ALA is beneficial for improving endothelial function and preventing atherosclerosis-related disease.16)
Therefore, in this study, we compared the effect of a stent coated with abciximab and ALA with that of bare metal stent in a porcine coronary overstretch restenosis model.
The animal study was approved by the Ethics Committee of Chonnam National University Medical School and Chonnam National University Hospital (CNU IACUC-H-2010-1), and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Study animals were female swine weighing 25-35 kg.
To prevent acute thrombosis after stenting, premedication with aspirin (100 mg/day) and clopidogrel (75 day/mg) was given for 7 days before the procedure.
On the procedure day, pigs were anesthetized with ketamine {20 mg/kg intramuscularly (IM)} and xylazine (2 mg/kg IM). They received IM ketamine every 30 minutes and supplemental oxygen continuously through an oxygen mask. Subcutaneous 2% lidocaine was administered at the cut-down site, left carotid artery was surgically exposed, and a 7 French sheath was inserted.
Continuous hemodynamic monitoring and surface electrocardiographic monitoring were maintained throughout the procedure. Heparin (5,000 units) was administered intravenously as a bolus prior to the procedure, the target coronary artery was engaged using standard 7 F guide catheters and control angiograms of both coronary arteries were performed using a nonionic contrast agent in two orthogonal views.
The pigs were randomized into two groups with 10 coronaries per group. One group received the control bare metal stents (3.0×18 mm, MAC®stent, AMG, Munich, Germany) and the second group received dual-coated stents (3.0×18 mm, abciximab and ALA coated MAC®stent). The stents were implanted in the proximal left anterior descending artery and proximal left circumflex artery and were deployed by inflating the balloon. The resulting stent-to-artery ratio was 1.3 : 1. Coronary angiograms were obtained immediately after stent implantation. All equipment was then removed and the carotid artery was ligated. All pigs continued to receive 100 mg of aspirin and 75 mg of clopidogrel daily until death.
Four weeks after stenting, the animals underwent follow-up angiography in the same orthogonal views. They were then sacrificed with an intercoronary injection of 20 mL of potassium chloride. The hearts were removed and the coronary arteries were pressure-perfusion fixed at 110 mmHg in 10% neutral buffered formalin overnight. The arteries were step-sectioned, processed routinely for light microscopy, and stained for histological analysis.
Histopathologic evaluation of each artery was performed by an experienced cardiovascular pathologist. The specimens were embedded in methylmethacrylate and sections were cut with a low-speed diamond wafer mounted to a Buehler Isomet saw (Buehler Ltd., Lake Bluff, IL, USA), leaving the stent wires intact in the cross sections to minimize potential artifacts caused by removal of the stent wires. Sections of 50 to 100 µm thickness were obtained at about 1 mm apart and stained with hematoxylin-eosin for histological analysis (Fig. 2). Measurements of the histopathologic sections were performed using a calibrated microscope, digital video imaging system, and microcomputer program (Visus 2000 Visual Image Analysis System, IMT Tech, CA, USA). Borders were manually traced for the lumen area, the area circumscribed by the internal elastic lamina, and the innermost border of the external elastic lamina (external elastic lamina area). Morphometric analysis of the neointimal area for a given vessel was calculated as the measured internal elastic lamina area minus the lumen area. Measurements were made on five cross-sections from the proximal and distal ends and the three midpoints of each stented segment. Histopathologic stenosis was calculated as 100×{1-(lesion lumen area/lesion internal elastic lamina area)}.
Arterial injury at each strut site was determined by the anatomic structures penetrated by each strut. A numeric value was assigned, as previously described by Schwartz et al.18) where 0=no injury, 1=break in the internal elastic membrane, 2=perforation of the media, and 3=perforation of the external elastic membrane to the adventitia. The average injury score for each segment was calculated by dividing the sum of injury scores by the total number of struts at the examined section.
The inflammation score for each individual strut was graded as follows: 0=no inflammatory cells surrounding the strut; 1=light, noncircumferential lymphohistiocytic infiltrate surrounding strut; 2=localized, moderate to dense cellular aggregate surrounding the strut noncircumferentially; and 3=circumferential dense lymphohistiocytic cell infiltration of the strut. The inflammation score for each cross section was calculated by dividing the sum of the individual inflammation scores by the total number of struts at the examined section.19) Inflammatory cell counts were normalized to neointimal hyperplasia area and percent area stenosis was compared between the two stent groups. Ordinal data for fibrin were collected on each stent section using a scale of 0 to 3 as previously reported (Fig. 3).20)
Statistical analysis was performed with the aid of the commercially available software (Statistical Package for the Social Sciences Version 15, Chicago, IL, USA). The data are presented as mean value±SD. Unpaired Student's t-test was used for the comparison of inflammatory cell counts normalized to injury score of the two stent groups. Analysis of variance was used for comparisons of the two stent groups. To examine the correlations between the measured histologic variables, regression analysis was applied for each set of measured variables. A p<0.05 was considered statistically significant.
Ten pigs were used in this study (10 pigs, 10 coronaries in each group). A bare metal stent (control) and a dual coated stent were implanted in proximal left anterior descending artery and proximal left circumflex artery by randomized in a pig.
Mortality for this study was zero. Quantitative coronary angiographies before and after stent implantation established the stent-to-artery diameter ratio as 1.33±0.06 for all 20 stented arteries. There was no significant difference in stent-to-artery ratio among the two stent groups.
On histopathologic analysis, various amounts of inflammatory cells and fibrin infiltrate surrounding on each strut section. In the neointima, most of the inflammatory cells in both groups were lymphohistiocytes. In contrast, eosinophils and plasma cells were not prominent. Carstair's fibrin stain for determining delayed arterial healing according to the discriminate fibrin score showed no difference between the two stent groups.
Significant correlations were found between the lymphohistiocyte count and the neointimal hyperplasia area (r=0.567, p<0.001) and between the lymphohitiocyte count and the percent area stenosis (r=0.476, p<0.001) (Fig. 4). There was no significant difference in the injury score between the two groups (1.44±0.59 in the dual-coated stent group vs. 1.33±0.66 in the control stent group, p=0.522), nor in the neointima area (1.55±0.82 mm2 in the dual-coated stent group vs. 1.40±0.86 mm2 in the control stent group, p=0.447) (Table 1). In the neointima, the lymphohistiocyte count was significantly lower in the dual-coated stent group compared with the control stent group (120±85 cells vs. 159±80 cells, p=0.048) (Fig. 5). There was no significant difference in the fibrin score between the two groups (0.16±0.34 in dual-coated stent group vs. 0.25±0.48 in the control stent group, p=0.446) (Fig. 3).
In our study, dual coating of a bare metal stent with abciximab and ALA by plasma polymerization of 1,2-DACH demonstrated no satisfactory inhibition of neointimal hyperplasia, but significantly lowered lymphohistiocyte counts compared with bare metal control stents.
Other studies have evaluated the effects of abciximab. In the EPIC trial, the incidence of further coronary events and the need for revascularization was reduced at 6 months post-stenting and beyond.13) Data from the EPISTENT trial also suggested a beneficial effect for abciximab on restenosis, although this was limited to patients with diabetes.21)
Other studies have shown that abciximab-coated stents reduce neointimal hyperplasia by inhibition of neointimal cell proliferation and extracellular matrix synthesis when compared with bare-metal stents in a porcine coronary restenosis model.22-24) Finally, two additional studies have reported that an abciximab-coated stent was feasible and produced significant inhibition of neointimal hyperplasia, showing a potential therapeutic benefit in the prevention of stent restenosis in human trials.25)26)
The results of the study presented here suggest that the possible mechanisms responsible for inhibition of neointimal hyperplasia by abciximab might be anti-platelet, anti-inflammatory, and anti-proliferative actions.
ALA exists endogenously and improves diabetic-induced endothelial dysfunction, probably due to antioxidant effects and direct free-radical scavenging properties.27) Moreover, ALA inhibits the inflammatory pathway and prevents neointimal hyperplasia after stenting in the carotid artery.28) Lim et al.29) have shown that an ALA-coated stent inhibits neointimal formation in a porcine coronary restenosis model. On histopathologic analysis, the neointimal area and the histopathologic area of stenosis were reduced in the ALA coated stent group compared with the control stent group. They hypothezied that by combining ALA and abciximab on a dual-coated stent, they could decrease the level of restenosis in the stented artery based on the anti-proliferative effects of ALA and anticoagulative effects of abciximab.29) Aditionally, delivery of these agents by loading them onto the stent might allow site-specific delivery of an appropriate dose over a prolonged period, thereby reducing potential unwanted systemic effects.
The most important mechanism of coronary stent restenosis is neointimal hyperplasia. However, the mechanism of the pathogenesis of neointimal hyperplasia is not well known. Previous studies have established that inflammation is a major contributor to restenosis after stenting and a significant correlation has been found between the inflammatory reaction and neointimal formation.30) By contrast in our study, a decrease in neointimal hyperplasia was not seen, in spite of the decreased inflammatory reaction seen in the group receiving the dual-coated stent when compared with the control group. We hypothesize that there are other, as yet unknown factors effecting neointimal hyperplasia as well as additional known factors on which the combination of abcixumab and ALA would have no effect. One of these known reasons is an increase in extracellular matrix, which is known to play an important role in neointimal hyperplasia after coronary artery stenting.30)
Based on this data, we conclude that ALA and abciximab suppressed the inflammatory reaction, but did not suppress the other causes of neointimal hyperplasia such as the increase in theextracellular matrix. In contrast to preclinical studies on abciximab and ALA, our study demonstrated that a stent coated with abciximab and ALA by plasma polymerization showed no satisfactory inhibition of neointimal hyperplasia compared with control stents in a porcine coronary restenosis model. We suspect that while the rapid release of abciximab and ALA suppressed the inflammatory reaction, the dosage of abciximab and ALA on the stent was not sufficient to inhibit neointimal hyperplasia.
Figures and Tables
Fig. 1
Concentration of released ALA and abciximab in the release medium from an ALA-abciximab-grafted stent as a function of time. ALA: alpha lipoic acid.

Fig. 2
Dual-coated stented porcine coronary arteries (A: H&E staining: ×20, B: methyl methacrylate staining: ×20) and control stented porcine coronary arteries (C: H&E staining: ×20, C: methyl methacrylate staining: ×20).
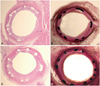
Fig. 3
The Carstair fibrin stain of the low- and high-power fields (magnitude, ×20, ×400) of fibrin infiltration in dual coating stent (A and B) and control stent (C and D).
Fig. 4
Correlations between the lymphohistiocyte count and neointimal hyperplasia area (A) and percent area stenosis (B).

Acknowledgments
This work was supported by a Grant-in-Aid for Strategy Technology Development Programs (No. 10030039) from the Korea Ministry of Knowledge and Economy.
References
1. Eeckhout E, Wijns W, Meier B, Goy JJ. Indications for coronary stent placement: the European view. Eur Heart J. 1999. 20:1014–1019.
2. Faxon DP, Spiro TE, Minor S, et al. Low molecular weight heparin in prevention of restenosis after angioplasty. Results of Enoxaparin Restenosis (ERA) Trial. Circulation. 1994. 90:908–914.
3. Hamm CW, Reimers J, Ischinger T, Rupprecht HJ, Berger J, Bleifeld W. A randomized study of coronary angioplasty compared with bypass surgery in patients with symptomatic multivessel coronary disease. German Angioplasty Bypass Surgery Investigation (GABI). N Engl J Med. 1994. 331:1037–1043.
4. Kent KM, Bentivoglio LG, Block PC, et al. Longterm efficacy of percutaneous transluminal coronary angioplasty (PTCA): report from the National Heart, Lung, and Blood Institute PTCA Registry. Am J Cardiol. 1984. 53:27C–31C.
5. Forrester JS, Fishbein M, Helfant R, Fagin J. A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. J Am Coll Cardiol. 1991. 17:758–769.
6. Austin GE, Ratliff NB, Hollman J, Tabei S, Phillips DF. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985. 6:369–375.
7. Bae Y, Jeong MH, Ahn YK, et al. Comparison of porcine coronary stent restenosis between MAC (Maximal Arterial Re-Creation) stent and Palmaz-Schatz stent. Korean Circ J. 1998. 28:89–96.
8. Giraldo AA, Esposo OM, Meis JM. Intimal hyperplasia as a cause of restenosis after percutaneous transluminal coronary angioplasty. Arch Pathol Lab Med. 1985. 109:173–175.
9. Ahn YK, Jeong MH, Kim JW, et al. Preventive effects of the heparin-coated stent on restenosis in the porcine model. Catheter Cardiovasc Interv. 1999. 48:324–330.
10. Hong YJ, Jeong MH, Lim SY, et al. Elevated preprocedural high-sensitivity C-reactive protein levels are associated with neointimal hyperplasia and restenosis development after successful coronary artery stenting. Circ J. 2005. 69:1477–1483.
11. The CAPTURE Investigators. Randomised placebo-controlled trial of abciximab before and during coronary intervention in refractory angina. Lancet. 1997. 349:1429–1435.
12. The EPILOG Investigators. Platelet glycoprotein IIb / IIIa blockade and low-dose heparin during percutaneous coronary revascularisation. N Engl J Med. 1997. 336:1689–1696.
13. Lefkovits J, Ivanhoe RJ, Califf RM, et al. Effects of platelet glycoprotein IIb/IIIa receptor blockade by a chimeric monoclonal antibody (abciximab) on acute and six-month outcomes after percutaneous transluminal coronary angioplasty for acute myocardial infarction. Am J Cardiol. 1996. 77:1045–1051.
14. Topol EJ, Ferguson JJ, Weisman HF, et al. Long term protection from myocardial ischaemic events in a randomised trial of brief integrin beta3 blockade with percutaneous coronary intervention. JAMA. 1997. 278:479–484.
15. Packer L, Witt EH, Tritschler HJ. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995. 19:227–250.
16. Packer L, Roy S, Sen CK. Alpha-lipoic acid: a metabolic antioxidant and potential redox modulator of transcription. Adv Pharmacol. 1997. 38:79–101.
17. Song SJ, Kim KS, Park YJ, Jeong MH, Ko YM, Cho DL. Preparation of a dual-drug-eluting stent by grafting of ALA with abciximab on a bare metal stent. J Mater Chem. 2009. 19:8135–8141.
18. Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992. 19:267–274.
19. Schwartz RS, Edelman E, Virmani R, et al. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv. 2008. 1:143–153.
20. Kolodgie FD, John M, Khurana C, et al. Sustained reduction of in-stent neointimal growth with the use of a novel systemic nanoparticle paclitaxel. Circulation. 2002. 106:1195–1198.
21. Lincoff AM. Potent complementary clinical benefit of abciximab and stenting during percutaneous coronary revascularization in patients with diabetes mellitus: results of the EPISTENT trial. Am Heart J. 2000. 139:S46–S52.
22. Kang KT, Jeong MH, Kim NH, et al. The inhibitory effect of platelet glycoprotein IIb/IIIa receptor blocker-coated stent on porcine coronary stent restenosis. Korean J Med. 2001. 60:314–323.
23. Park OY, Jeong MH, Kim JH, et al. The inhibitory effects of platelet glycoprotein IIb/IIIa receptor blocker-coated stent on neointima formation and inflammatory response in porcine coronary stent restenosis. Korean Circ J. 2003. 33:439–445.
24. Hong YJ, Jeong MH, Kim W, et al. The effects of abciximab (Reopro(r))-coated stents on extracellular matrix synthesis and apoptosis. Korean Circ J. 2005. 35:290–301.
25. Hong YJ, Jeong MH, Kim W, et al. Effect of abciximab-coated stent on in-stent intimal hyperplasia in human coronary arteries. Am J Cardiol. 2004. 94:1050–1054.
26. Kim W, Jeong MH, Kim KH, et al. The clinical results of a platelet glycoprotein IIb/IIIa receptor blocker (abciximab: Reo-Pro)-coated stent in acute myocardial infarction. J Am Coll Cardiol. 2006. 47:933–938.
27. Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III Study Group. Alpha-Lipoic Acid in Diabetic Neuropathy. Diabetes Care. 1999. 22:1296–1301.
28. Sung MJ, Kim W, Ahn SY, et al. Protective effect of alpha-lipoic acid in lipopolysaccharide-induced endothelial fractalkine expression. Circ Res. 2005. 97:880–890.
29. Lim SY, Bae EH, Jeong MH, et al. The effect of alpha lipoic acid in a porcine in-stent restenosis model. J Cardiol. 2009. 54:375–385.
30. Park OY, Jeong MH, Kim JH, et al. The role of extracellular matrix within the neointima in a porcine coronary stent restenosis model. Korean Circ J. 2003. 33:121–129.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download