Introduction
Coronary embolism as a cause of myocardial infarction is an uncommon finding.1) Coronary embolism should be suspected in the context of a valvular prosthesis, dilated cardiomyopathy, rheumatic valvular disease, intracardiac shunts, infective endocarditis, chronic atrial fibrillation and hypercoaguable states including pregnancy.2) This list also includes distal embolization during primary percutaneous intervention for acute myocardial infarction (AMI). Percutaneous coronary intervention (PCI), thrombolysis and surgical treatment are management options available.1)
Here we report a case of AMI due to migration of coronary emboli from a left main coronary artery (LMA) thrombus during coronary angiography (CAG).
Case
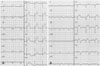
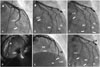
A 48-year-old male presented at our hospital with typical chest pain. His past medical history was unremarkable. His coronary risk factors only included "current smoker". On physical examination, he had a systolic blood pressure of 120 mmHg and a heart rate of 62 beats per minute. An initial electrocardiogram (ECG) showed no definite ST segment change and sinus rhythm (Fig. 1A). Initial creatine kinase-MB and cardiac troponin I levels were in the normal range. Transthoracic echocardiography showed no regional wall motion abnormality and normal left ventricular function. We administered aspirin 300 mg for 1 day. CAG revealed a filling defect of the distal left anterior descending artery (dLAD), distal left circumflex artery (dLCX) and LMA (Fig. 2A, B). We decided to aspirate the LMA thrombus and to do PCI at the dLAD and dLCX. A guiding catheter was positioned in the left coronary artery ostium. At that time, he complained of aggravating chest pain. On CAG, the distal region of the 1st diagonal branch (D1) and the obtuse marginal branch (OM) were not visualized (Fig. 2C). We thought that this event had developed due to coronary embolization that resulted from the migration of thrombi into the LMA during CAG. Intravascular ultrasonography showed the LMA thrombus (Fig. 2D). We tried to aspirate the thrombus in the LMA using a guiding catheter and to do balloon angioplasty in the dLAD and dLCX. Balloon angioplasty was successful only for D1 (Fig. 2E). However, the dLAD, dLCX and OM had Thrombolysis In Myocardial Infarction (TIMI) grade 1 flow in spite of repeated balloon angioplasty. Applied platelet glycoprotein IIb/IIIa receptor antagonist (Abcimixab, Reopro®) and balloon angioplasty of the dLAD, OM and dLCX were done sequentially. However, filling defects in the dLAD, OM and dLCX remained (Fig. 2F).
We cared for the patient in the cardiovascular care unit. Creatine kinase-MB and cardiac troponin I levels were elevated to 349.4 ng/mL and 40.91 ng/mL, respectively. Follow up ECG showed a 3 mm ST segment elevation at leads II, III, aVF and V3-5 after angiography (Fig. 1B). We thought that the AMI developed due to distal embolization of the LMA thrombus during angiography. Laboratory profiles associated with hypercoaguability such as lupus anticoagulant, protein C activity 102% (NL 70-130%), protein S activity 74% (NL 73.7-146.3%), D-dimer 0.36 µg/mL (NL 0.0-0.4 µg/mL), antithrombin III 91% (NL 80-120%) and homocysteine 12.2 µmol/L (NL 5.0-15.0 µmol/L) were in the normal range.
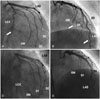
After 3 days of intravenous unfractionated heparin (25,000 IU/day), follow-up CAG showed only slight resolution of the filling defect and only in the dLAD, not in the OM and dLCX (Fig. 3A and B). He had no chest pain and was discharged on triple antiplatelet agent. After 1 year of this treatment, he experienced no chest pain and, on follow up CAG, had no significant stenosis or intracoronary filling defects (Fig. 3C and D).
Discussion
Although rupture of atherosclerotic plaques and intracoronary thrombus formation is widely accepted as the main pathophysiological cause for the development of AMI, four to seven percent of patients with AMI do not have underlying coronary artery disease.1)3) Coronary embolism as a cause of myocardial infarction is an uncommon but important entity in terms of both etiology and treatment.1) Coronary embolism leading to myocardial infarction was first reported by Virchow in 1856.4) Previous cases of coronary emboli have been reported in association with valvular prosthesis, dilated cardiomyopathy, rheumatic valvular disease, intracardiac shunts, infective endocarditis, chronic atrial fibrillation and hypercoaguable states including pregnancy.2) In addition, primary percutaneous intervention for AMI may be complicated by distal embolization of plaque or thrombotic debris, with infarct extension.5) Henriques et al.6) reported distal embolization in patients treated with primary angioplasty is visible on the coronary angiogram in 15.2% of patients. In an unusual case, van Gaal et al.7) showed that a localized thrombus at the site of plaque rupture may embolise, causing coronary occlusion downstream in the dependent vascular territory, as in our case.
Appropriate treatment of coronary embolism remains a therapeutic challenge. The strategy of intervention varies according to the acuity of the clinical presentation. If coronary embolism results in myocardial infarction associated with ST-elevation, various interventional procedures including Fogarty maneuvers, sole balloon angioplasty, stenting or thrombus aspiration have been suggested. However, in the case of non-ST-elevation myocardial infarction, there is no conclusive evidence favouring an interventional or conservative strategy.8) These techniques are particularly advocated in the setting of acute coronary syndromes.9)
In particular, coronary embolism associated with an LMA thrombus is associated with increased and extensive myocardial damage and adverse clinical outcomes such as sudden death or AMI.10) In some cases, aspiration thrombectomy has been successfully used for LMA thrombus. However, indications for this therapeutic approach are determined by coronary anatomy, clinical stability and the hemodynamic condition of the patient.10) Ahn et al.11) reported intracoronary thrombosis treated with stent and abciximab.
We demonstrated that AMI developed due to coronary embolism from an LMA thrombus without any apparent cause of the systemic embolism, which progressed with angiography. Therefore, we thought that embolization of an LMA thrombus should be considered in cases where, on CAG, there are multiple coronary artery filling defects.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download