Abstract
Background
Weakness of cervical extensor muscles causes loss of cervical lordosis, which could also cause neck pain. The aim of this study was to investigate the impact of fat infiltration in cervical extensor muscles on cervical lordosis and neck pain.
Methods
Fifty-six patients who suffered from neck pain were included in this study. Fat infiltration in cervical extensor muscles was measured at each level of C2–3 and C6–7 using axial magnetic resonance imaging. The visual analogue scale (VAS), 12-Item Short Form Health Survey (SF-12), and Neck Disability Index (NDI) were used for clinical assessment.
Results
The mean fat infiltration was 206.3 mm2 (20.3%) at C2–3 and 240.6 mm2 (19.5%) at C6–7. Fat infiltration in cervical extensor muscles was associated with high VAS scores at both levels (p = 0.047 at C2–3; p = 0.009 at C6–7). At C2–3, there was a negative correlation between fat infiltration of the cervical extensor muscles and cervical lordosis (r = −0.216; p = 0.020). At C6–7, fat infiltration in the cervical extensor muscles was closely related to NDI (p = 0.003) and SF-12 (p > 0.05). However, there was no significant correlation between cervical lordosis and clinical outcomes (VAS, p = 0.112; NDI, p = 0.087; and SF-12, p > 0.05).
The posterior extensor muscles of the cervical spine help stabilize the head and maintain the alignment of the cervical spine and function in neck motion. Panjabi et al.1) estimated that the neck musculature is about 80% responsible for the mechanical stability of the cervical spine and the osteoligamentous system is for the remaining 20%. Atrophy and fat infiltration of lumbar extensor muscles are well-known risk factors of lumbar degenerative kyphosis. Furthermore, many articles have reported the relationship between fat infiltration of lumbar extensor muscles and chronic back pain.23456) On the other hand, few studies have addressed the correlation between cervical spine muscles and clinical scales. In addition, most of such studies focused on patients with acute or chronic whiplash injury.78) Therefore, we set out to investigate the impact of fat infiltration in cervical extensor muscles on cervical lordosis and neck pain under the hypothesis that fat infiltration of cervical extensor muscles could induce muscle weakness, neck pain, and loss of cervical lordosis.
The subjects were 56 patients (28 females and 28 males) who visited Kwangju Christian Hospital between October 2013 and December 2014 with a complaint of cervicalgia lasting for 3 months or more. The patients underwent cervical spine lateral radiography and cervical spine magnetic resonance imaging (MRI). Their mean age was 39.7 ± 9.0 years and the average body mass index (BMI) was 23.8 ± 1.6 kg/m2 (Table 1). The following subjects were excluded from this study: patients who had a history of trauma, previous surgery anamnesis, or inflammatory disease (e.g., ankylosing spondylitis and rheumatoid arthritis); patients with radiculopathy symptoms complaining of referred pain to upper limbs.
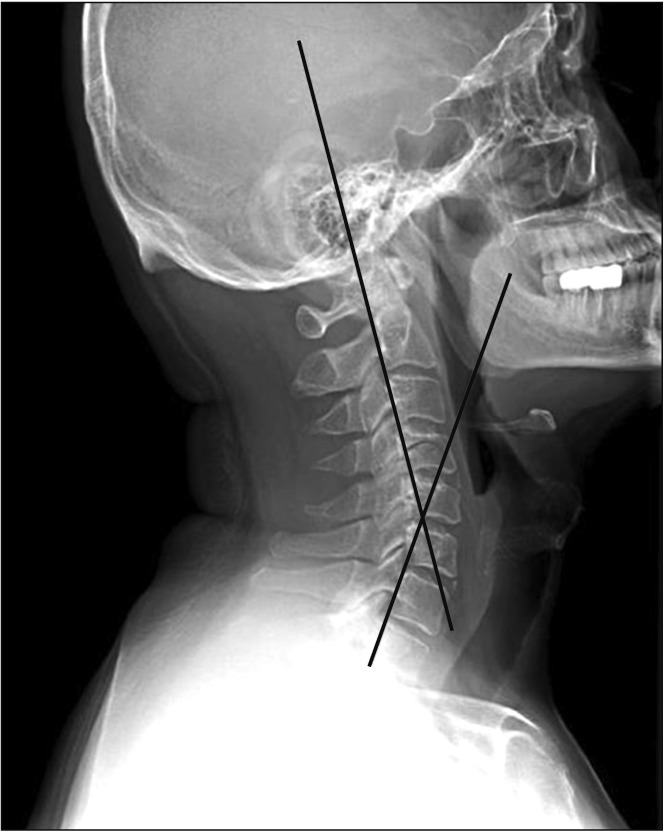
The cervical spine lateral radiograph was taken in all patients in a standing position using the posterior tangent technique proposed by Harrison et al.9) Cervical lordosis was measured between C2 and C7 vertebral bodies on the cervical spine lateral radiograph (Fig. 1). In order to evaluate the cervical extensors' muscle amount and fat infiltration, T2-weighted axial MRI scans were obtained using 1.5T Signa Excite GE (General Electric, Milwaukee, WI, USA) MRI scanner. Imaging analysis was conducted using DICOM files saved in picture archiving and communication system (PACS) with Piview (Infinitt Healthcare, Seoul, Korea). With use of the pseudo-coloring technique proposed by Lee et al.,10) the cervical extensor muscle amount and fat infiltration were assessed at the upper cervical segment (C2–3 cervical intervertebral disc center) and lower cervical segment (C6–7 cervical intervertebral center) each (Fig. 2). In addition, the severity of intervertebral disc disease was assessed according the cervical intervertebral disc degeneration grade proposed by Miyazaki et al.11) (Table 2). The grade of intervertebral disc degeneration in a patient was determined by calculating the mean of the sum of cervical intervertebral disc degeneration grades of six segments from C2–3 through C7–T1 intervertebral discs (Fig. 3).
The visual analogue scale (VAS) score, 12-Item Short Form Health Survey (SF-12), and Neck Disability Index (NDI) were assessed during patients' visit for cervical spine MRI due to neck pain. The VAS is a straight line with one end meaning “no pain” (left) and the other end meaning “worst pain imaginable” (right). Patients were asked to mark a point on the line that best matches their pain intensity. The SF-12 is a 12-item questionnaire used to assess generic health outcomes from the patient's perspective.12) The values in each domain of the SF-12 contribute to physical and mental values (physical component summary [PCS] and mental component summary [MCS]), which provide glimpses into mental and physical functioning and overall health-related quality of life. PCS and MCS are computed using the scores of 12 questions and range from 0 to 100, where 0 indicates the lowest level of health and 100 indicates the highest level of health.
The NDI is a self-administered instrument for assessing disability due to neck pain of mechanical origin.13) It consists of 10 items and each item has six different assertions expressing progressive levels of pain or limitation in activities. Item scores range from 0 (no pain or limitation) to 5 (as much pain as possible or maximal limitation), and the total NDI score ranges from 0 to 50 points. Higher scores indicate greater disability.
We conducted this study in compliance with the principles of the Declaration of Helsinki. The protocol of this study was reviewed and approved by the Institutional Review Board of Kwangju Christian Hospital (IRB No. KCH-M-2017-11-014), and informed consent was waived. Statistical analysis was conducted using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). The relationship of the degree of fat infiltration with clinical scales and cervical lordosis as well as the relationship between clinical scales and cervical lordosis was determined by correlation analysis.
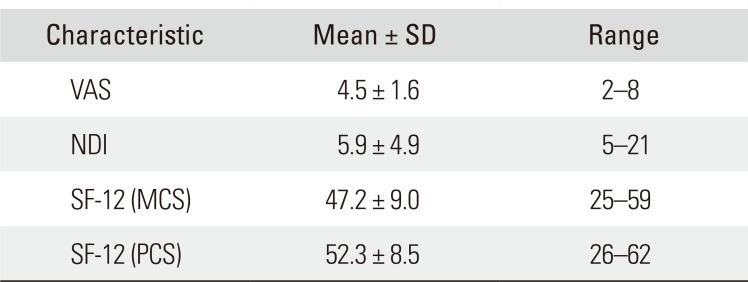
On radiographic results, the mean fat infiltration of the cervical extensor muscles was 206.3 ± 48.7 mm2 at C2–3 and 240.6 ± 55.9 mm2 at C6–7 segment. The average cervical lordotic angle was 22.1° ± 7.1°. The mean grade of intervertebral disc degeneration was 2.3 ± 0.5 (Table 3). On clinical results, the mean VAS score was 4.5 ± 1.6 and the mean NDI was 5.9 ± 4.9. The mean SF-12 MCS score and SF-12 PCS score were 47.2 ± 9.0 and 52.3 ± 8.5, respectively (Table 4).
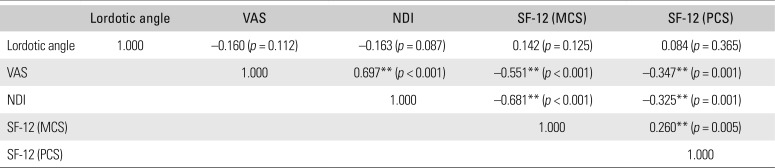
Increased fat infiltration in the cervical extensor muscles was significantly associated with higher VAS scores (C2–3, p = 0.047; C6–7, p = 0.009) (Table 5). There was negative correlation between cervical lordosis and fat infiltration of the cervical extensor muscles at C2–3 (r = −0.216, p = 0.020) (Table 6). Greater fat infiltration in cervical extensor muscles at C6–7 was related to poorer clinical results of NDI (r = 0.279, p = 0.003) and SF-12 (MCS: r = −0.283, p = 0.002; PCS: r = −0.181, p = 0.049) (Table 5). However, there was no significant correlation between cervical lordosis and clinical scales (VAS, p = 0.112; NDI, p = 0.087; SF-12, p > 0.05) (Table 7). Fat infiltration of cervical extensor muscles did not relate to the cervical intervertebral disc degeneration grade, age, and BMI (p > 0.05) (Table 6).
Dysfunction of extensor muscles of the cervical spine may result in neck pain, cervical malalignment, and a reduced range of motion.1415) Fat infiltration seems to be a late stage symptom of muscular degeneration.61617) However, there was some controversy about the influence of fat infiltration of cervical extensor muscles on cervical lordosis and neck pain. We therefore questioned whether fat infiltration of the cervical extensor muscles could induce the loss of cervical lordosis and cervical extensor muscles may affect clinical dysfunction including neck pain.
The possible causes of intramuscular fat infiltration include disuse, degenerative changes of the muscle, minor damage or irritation to the nerves, and demyelination of neural tissue consecutive to acute inflammation.18192021) On the lumbar spine, Lee et al.10) reported significant correlation between lumbar extensors' fat infiltration and flat back syndrome.22) On the cervical spine, Hogan et al.23) reported a case of an acute progressive cervical kyphotic deformity after injecting botulinum into the cervical extensor. This acute cervical kyphotic deformity resulted from cervical extensor muscle weakness by blocking the calcium ion channel of the botulinum's mechanism. In this study, fat infiltration in the upper cervical extensor muscles had relevance to the loss of cervical lordosis.
The degenerative changes of extensor muscles and proportion of fat infiltration were associated with chronic back pain in the lumbar spine.246) In a cervical spine study, Hallgren et al.24) reported that there was significant atrophy and fat infiltration of the cervical extensor in a patient complaining of chronic neck pain; on the other hand, Elliott et al.825) reported that chronic neck pain did not develop gradually although there was significant increase of cervical extensor fat infiltration by chronic whiplash injury. In this study, increased fat infiltration in the cervical extensor muscles was significantly associated with higher VAS scores. In particular, more fat infiltration in lower cervical extensor muscles was related to poorer neck function and neck pain. Likewise, increased fat infiltration of cervical extensor muscles accompanying atrophy and weakness can induce loss of cervical lordosis. In addition, an axial load to the head and neck transmitted downward can induce excessive tension in the posterior neck musculature and muscles near the upper back, causing pain around the neck. An axial load to the head and neck transferred forward can cause not only persistent tensile force on the posterior neck musculature but also fatigue and weakness of the cervical extensors. These processes lead to vicious cycle and result in a flat cervical spine.2627) Besides, although increased fat infiltration of cervical extensor muscles might be related to the aging process and obesity, it showed no significant correlation with age and BMI, and cervical intervertebral disc degeneration grade in this study.
This study has some limitations. First, it is a cross-sectional study that does not reflect dynamic changes of clinical results including neck pain and cervical lordosis. Second, the sample size was small and the neck pain could have been due to other causes, such as intervertebral disc disease and myofasciitis. However, despite these limitations, this study introduces potential areas that warrant future research. To achieve more reliability of our findings, it is necessary to conduct prospective large cohort studies regarding the influence of fat infiltration of cervical extensors on cervical lordosis and neck pain in a general population in the future.
In conclusion, these results suggest that fat infiltration in the upper cervical extensor muscles has relevance to the loss of cervical lordosis and fat infiltration in the lower cervical extensor muscles is associated with cervical functional disability. Therefore, we recommend muscle strengthening exercises to prevent fat infiltration of cervical extensor muscles, which could also be effective for relief of neck pain as well as maintenance of cervical lordosis.
ACKNOWLEDGEMENTS
This article was presented at the 32th Spring Congress Korean Society of Spine Surgery in Pyeongchang by Dr. Choong-Young Kim in 2015.
References
1. Panjabi MM, Cholewicki J, Nibu K, Grauer J, Babat LB, Dvorak J. Critical load of the human cervical spine: an in vitro experimental study. Clin Biomech (Bristol, Avon). 1998; 13(1):11–17.

2. Campbell WW, Vasconcelos O, Laine FJ. Focal atrophy of the multifidus muscle in lumbosacral radiculopathy. Muscle Nerve. 1998; 21(10):1350–1353. PMID: 9736071.

3. Kader DF, Wardlaw D, Smith FW. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol. 2000; 55(2):145–149. PMID: 10657162.

4. Alaranta H, Tallroth K, Soukka A, Heliovaara M. Fat content of lumbar extensor muscles and low back disability: a radiographic and clinical comparison. J Spinal Disord. 1993; 6(2):137–140. PMID: 8504225.
5. van Tulder MW, Assendelft WJ, Koes BW, Bouter LM. Spinal radiographic findings and nonspecific low back pain: a systematic review of observational studies. Spine (Phila Pa 1976). 1997; 22(4):427–434. PMID: 9055372.
6. Kjaer P, Bendix T, Sorensen JS, Korsholm L, Leboeuf-Yde C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med. 2007; 5:2. PMID: 17254322.

7. Elliott J, Sterling M, Noteboom JT, Treleaven J, Galloway G, Jull G. The clinical presentation of chronic whiplash and the relationship to findings of MRI fatty infiltrates in the cervical extensor musculature: a preliminary investigation. Eur Spine J. 2009; 18(9):1371–1378. PMID: 19672633.

8. Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine (Phila Pa 1976). 2006; 31(22):E847–E855. PMID: 17047533.
9. Harrison DE, Harrison DD, Cailliet R, Troyanovich SJ, Janik TJ, Holland B. Cobb method or Harrison posterior tangent method: which to choose for lateral cervical radiographic analysis. Spine (Phila Pa 1976). 2000; 25(16):2072–2078. PMID: 10954638.
10. Lee JC, Cha JG, Kim Y, Kim YI, Shin BJ. Quantitative analysis of back muscle degeneration in the patients with the degenerative lumbar flat back using a digital image analysis: comparison with the normal controls. Spine (Phila Pa 1976). 2008; 33(3):318–325. PMID: 18303466.
11. Miyazaki M, Hong SW, Yoon SH, Morishita Y, Wang JC. Reliability of a magnetic resonance imaging-based grading system for cervical intervertebral disc degeneration. J Spinal Disord Tech. 2008; 21(4):288–292. PMID: 18525490.

12. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996; 34(3):220–233. PMID: 8628042.
13. Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991; 14(7):409–415. PMID: 1834753.
14. McPartland JM, Brodeur RR, Hallgren RC. Chronic neck pain, standing balance, and suboccipital muscle atrophy: a pilot study. J Manipulative Physiol Ther. 1997; 20(1):24–29. PMID: 9004119.
15. Anderson JS, Hsu AW, Vasavada AN. Morphology, architecture, and biomechanics of human cervical multifidus. Spine (Phila Pa 1976). 2005; 30(4):E86–E91. PMID: 15706328.

16. Shahidi B, Parra CL, Berry DB, et al. Contribution of lumbar spine pathology and age to paraspinal muscle size and fatty infiltration. Spine (Phila Pa 1976). 2017; 42(8):616–623. PMID: 27517512.

17. Shahidi B, Hubbard JC, Gibbons MC, et al. Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. J Orthop Res. 2017; 35(12):2700–2706. PMID: 28480978.

18. Nukada H, McMorran PD, Shimizu J. Acute inflammatory demyelination in reperfusion nerve injury. Ann Neurol. 2000; 47(1):71–79. PMID: 10632103.

19. Andary MT, Hallgren RC, Greenman PE, Rechtien JJ. Neurogenic atrophy of suboccipital muscles after a cervical injury: a case study. Am J Phys Med Rehabil. 1998; 77(6):545–549. PMID: 9862543.
20. Fleckenstein JL, Watumull D, Conner KE, et al. Denervated human skeletal muscle: MR imaging evaluation. Radiology. 1993; 187(1):213–218. PMID: 8451416.

21. Dulor JP, Cambon B, Vigneron P, et al. Expression of specific white adipose tissue genes in denervation-induced skeletal muscle fatty degeneration. FEBS Lett. 1998; 439(1-2):89–92. PMID: 9849884.

22. Maughan RJ. Relationship between muscle strength and muscle cross-sectional area: implications for training. Sports Med. 1984; 1(4):263–269. PMID: 6568749.
23. Hogan KA, Manning EL, Glaser JA. Progressive cervical kyphosis associated with botulinum toxin injection. South Med J. 2006; 99(8):888–891. PMID: 16929888.

24. Hallgren RC, Greenman PE, Rechtien JJ. Atrophy of suboccipital muscles in patients with chronic pain: a pilot study. J Am Osteopath Assoc. 1994; 94(12):1032–1038. PMID: 7852102.
25. Elliott J, Sterling M, Noteboom JT, Darnell R, Galloway G, Jull G. Fatty infiltrate in the cervical extensor muscles is not a feature of chronic, insidious-onset neck pain. Clin Radiol. 2008; 63(6):681–687. PMID: 18455560.

Fig. 2
Pseudo-coloring program was applied on the C2–3 axial image. (A) The muscle tissue on the magnetic resonance imaging scan is colored gray. (B) The area of pseudo-color masking of region of interest was obtained.
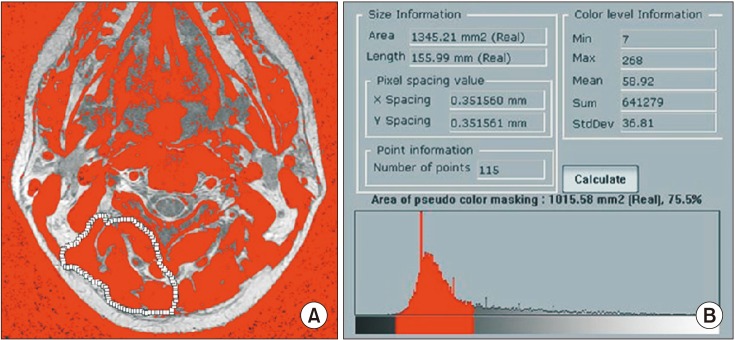
Fig. 3
Degeneration grades of the cervical intervertebral discs in magnetic resonance imaging are as follows: C2–3, grade I; C3–4, grade II; C4–5, grade II; C5–6, grade III; C6–7, grade III; and C7–T1, grade II.
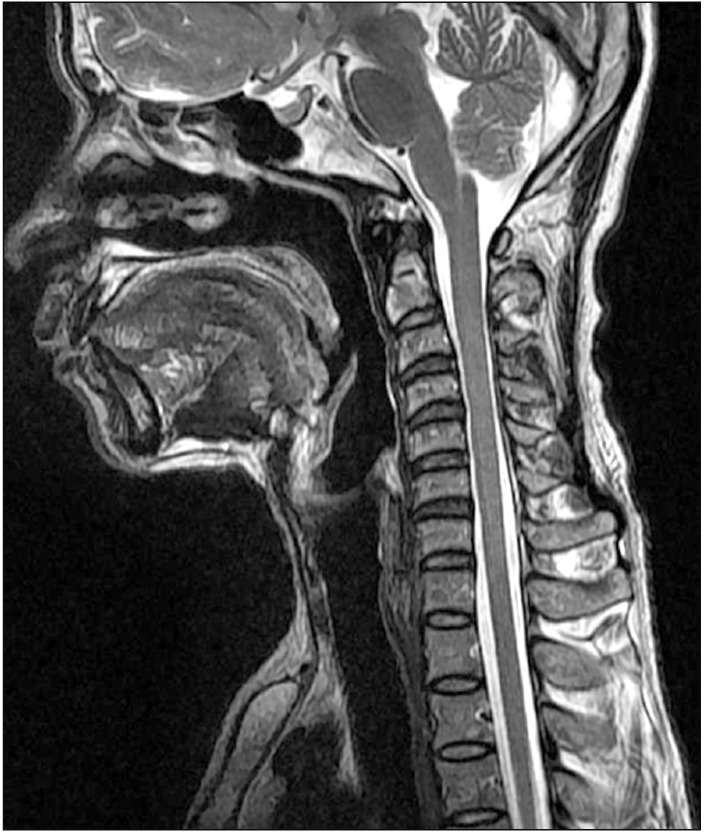
Table 1
Demographic Characteristics of the Study Subjects
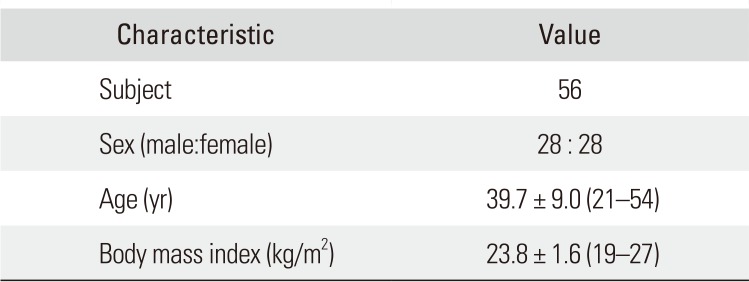
| Characteristic | Value |
|---|---|
| Subject | 56 |
| Sex (male:female) | 28 : 28 |
| Age (yr) | 39.7 ± 9.0 (21–54) |
| Body mass index (kg/m2) | 23.8 ± 1.6 (19–27) |
Table 2
Grading System for Cervical Intervertebral Disc Degeneration
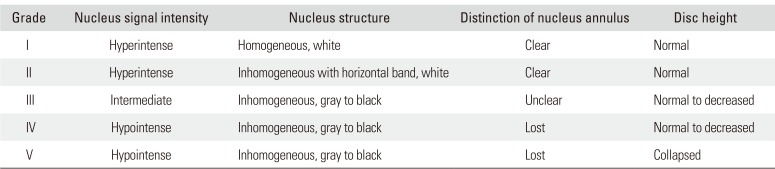
Table 3
Radiographic Measurements of the Subjects
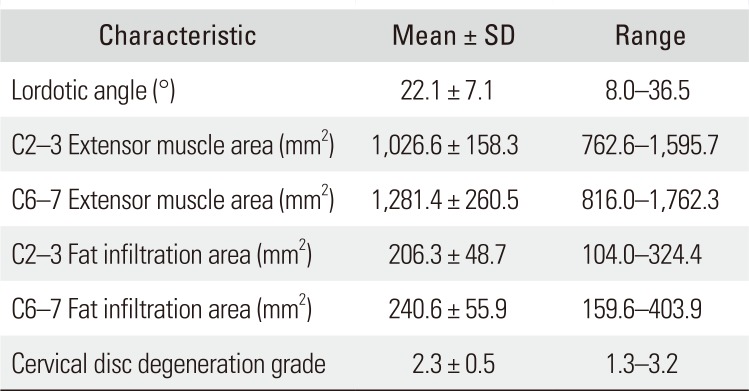
Table 4
Clinical Scales of the Subjects
| Characteristic | Mean ± SD | Range |
|---|---|---|
| VAS | 4.5 ± 1.6 | 2–8 |
| NDI | 5.9 ± 4.9 | 5–21 |
| SF-12 (MCS) | 47.2 ± 9.0 | 25–59 |
| SF-12 (PCS) | 52.3 ± 8.5 | 26–62 |
Table 5
Correlation Coefficient (r) between Clinical Scales and Fat Infiltration of Cervical Extensor Muscle
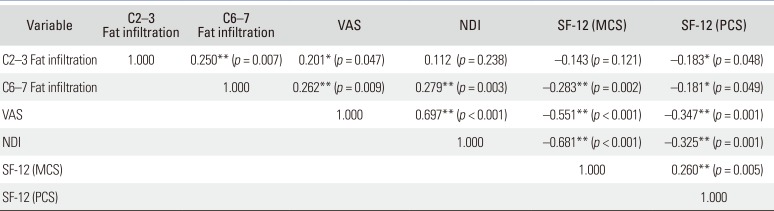
Table 6
Correlation Coefficient (r) among Cervical Lordotic Angle, Cervical Disc Degeneration Grade, Age, BMI, and Fat Infiltration of Cervical Extensor Muscle
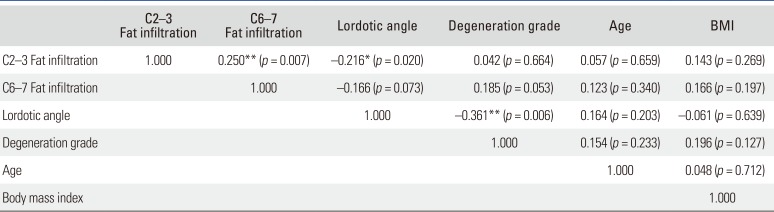
Table 7
Correlation Coefficient (r) between Cervical Lordotic Angle and Clinical Scales




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download