Abstract
Purpose
We investigated the feasibility of using surgical clips as markers for tumor localization and their effect on the imaging evaluation of treatment responses after neoadjuvant chemotherapy (NAC).
Methods
A total of 16 breast cancers confirmed by needle biopsy in 15 patients were included in this study from October 2012 to June 2014. Under ultrasonography (US)-guidance, the surgical clips were placed prior to NAC. Additional mammography, breast US, and breast magnetic resonance examinations were performed within 10 days before surgery. The time period from marker insertion to operation date was documented. Images acquired via the three modalities were evalu-ated for the following parameters: location of clip, clip migration (>1 cm), the presence of complications from clip placement, and the effect of clips on the assessment of treatment.
Results
The mean time period was 128.6±34.4 days (median, 132.0 days) from the date of clip insertion to the date of surgery. The mean number of inserted clips was 2.3±0.7 (median, 2.0). Clip migration was not visualized by imaging in any patient, and there were no complications reported. Surgical clips did not negatively affect the assessment of treatment responses to NAC.
Conclusion
Surgical clips may replace commercial tissue markers for tumor localization in breast cancer patients undergoing NAC without migration. Surgical clips are well tolerated and safe for the patient, easily visualized on imaging, do not interfere with treatment response, and are cost-effective.
Neoadjuvant chemotherapy (NAC) has been the accepted standard of care for patients with operable or inoperable breast cancers. The benefits of NAC performed prior to surgery are as follows: (1) reduction of mortality; (2) improvement of surgical options, such as conversion to breast-conserving surgery (BCS) in operable patients, as well as surgery in previously inoperable patients; and (3) early collection of information on the treatment response and tumor biology of the breast cancer [1,2,3,4].
The placement of radiopaque markers has proven to be helpful and safe for tumor localization in patients undergoing NAC and BCS [2]. Many types of commercial breast markers have been developed and are widely used prior to NAC, especially in the United States. Until recently, none of these markers had been legally allowed for use in South Korea due to the lack of approval from the Korean Food and Drug Administration (KFDA), although clinicians have long advocated for a reliable, safe, and less expensive radiopaque marker. As new chemotherapeutic agents have been developed, patients who have undergone NAC have shown a positive response (approximately 80%). Sometimes a dramatic pathologic complete response (pCR) can be achieved, but results vary with the treatment regimen (6.0%-32.9%) [3,5,6]. For these reasons, international breast cancer specialist panels in 2006 and 2010 referred to the importance of the radiopaque clip [7]. The placement of radiopaque markers are essential for patients with NAC and BCS because a dramatic pCR in a patient with a nonradiopaque marker would not allow the surgeon to accurately locate and excise any residual cancerous tissue, or reconstruct the breast with a satisfactory cosmetic result [2,6].
Titanium surgical clips are readily available and have been proven to be safe in patients. Titanium clips are relatively less expensive than commercial breast markers, and both are composed mainly of titanium. Surgical clips are considered safer than commercial breast markers because they are removed after surgery. For this reason, we studied the use of surgical clips for tumor localization in breast cancer patients who were scheduled for surgery after preoperative NAC.
The purpose of our study was to investigate the feasibility of using surgical clips as markers for tumor localization and their influence on the imaging assessment of treatment responses after NAC.
The Institutional Review Board approved this retrospective study (KBSMC 2014-01-123-002) and required neither patient approval nor patient informed consent for the review of ima-ges and records. However, all patients in this study had agreed to the procedure and prior informed consent for the procedure was obtained.
Between October 2012 and June 2014, 94 patients at our institution received NAC for breast cancer. The decision to perform clip placement was made subjectively by the attending surgeon, and patients who agreed to the procedure were enrolled in this study. A total of 21 patients underwent preoperative ultrasonography (US)-guided surgical clip insertion to accurately localize the malignant lesion before their scheduled preoperative NAC. After a course of NAC, patients underwent mammography, US, and magnetic resonance imaging (MRI) to evaluate chemotherapy response before the elective surgery. Of these patients, we excluded three because they had not yet undergone surgery during the study period. Another three patients were excluded because they had no mammography or MRI records during the time from clip insertion to operation. This study ultimately included 16 malignant lesions in 15 patients (mean age, 46.1±7.6 years) who wanted to undergo BCS after US-guided surgical clip insertion prior to NAC. The NAC regimen was four to six cycles of combined adriamycin, docetaxel, 5-fluorouracil, and cyclophosphamide.
All mammographic studies were performed with standard craniocaudal and mediolateral oblique views of both breasts on a full field digital mammography unit (Lorad Selenia; Hologic, Danbury, USA). All patients underwent real-time gray-scale US scans (Philips iU22 platform, Philips Healthcare, Bothell, USA; Aixplorer; SuperSonic Imagine, Aix-en-Provence, France) with two orthogonal planes using a 10-12 MHz linear transducer and dynamic contrast-enhanced MRI (Achieva; Philips Medical System, Best, The Netherlands) examinations using a 3.0-T system and a dedicated 7-channel SENSE breast coil. Unenhanced T2-weighted (W) turbo spin echo axial im-ages (TR/TE: 3790/100, 332×316 matrix; field of view: 200×340 mm; slice thickness: 3 mm, 1 mm gap), T1-W spin echo axial images (TR/TE: 620/10, 332×332 matrix; field of view: 200×340 mm; slice thickness: 3 mm, 1 mm gap), dynamic contrast-enhanced examination using a fat-suppressed T1-W 3D fast field echo sequence (TR/TE: 7.0/3.5, 452×410 matrix; field of view: 340×340 mm; slice thickness: 2 mm, no gap), delayed axial T1-W spin echo images (TR/TE: 532/10, 448×378 matrix; field of view: 380×380 mm; slice thickness: 5 mm, 2.5 mm gap), and maximum intensity projection images were obtained. All MRI images were evaluated using a commercial computer-aided diagnosis system (CADstream, version 5.4; Merge Healthcare, Chicago, USA).
The initial histological diagnosis of malignant lesion was made through a US-guided 14-gauge core needle biopsy (CNB) at our institution or at an outside hospital. If a CNB specimen slide was prepared at an outside clinic, it was reviewed by pathologists at our institution. US-guided fine needle aspiration was performed if suspicious axillary nodes were found on US.
We prepared surgical clips with a disposable clip applier (LigaClip MCA MSM20, Ethicon Endo-Surgery, Somerville, USA; Premium Surgiclip M-9.75, Covidien, Mansfield, USA) for patients scheduled for NAC and who had agreed to surgical clip insertion (Figure 1A). A short skin incision was made using local anesthesia under aseptic conditions. A14/16-gauge coaxial guiding needle (TSK Stericut; TSK Laboratory, Tochigi, Japan) was inserted into the center of the malignancy, and the inner stylet was removed under US guidance. One to two surgical clips were passed through the inserted introducer, and the inner stylet was reinserted to complete the clip placement (Figure 1B). We confirmed the location of the clips by US immediately after clip insertion. If there were the multiple or large breast cancers, additional clips were placed for lesion extent bracketing as per the radiologist's judgment. One of three experienced breast radiologists (2-20 years of experience) performed these procedures in consensus. Postprocedural mammography was performed to confirm objectively the appropriate location of the inserted clips.
Final histopathological results from both the initial biopsy and surgery were reviewed. Final pathological types of breast cancer, tumor (T) stages, and immunochemical markers, including the estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2) were also evaluated.
Follow-up mammography, US, and MRI were performed within the 10-day period before elective surgery to evaluate treatment response after NAC. After surgical excision, specimen mammography was also done to evaluate clip retrieval and to assess specimen margin (Figure 2). A pathologist confirmed the results.
We calculated the time interval from clip insertion to postprocedural mammography, follow-up preoperative mammo-graphy, US and MRI, and BCS. Each interval was expressed by mean±standard deviation (days) and median values. We also recorded the number of inserted clips. Two experienced breast radiologists (I.Y. and SH.C.) retrospectively reviewed the medical records, and all images from the time of clip insertion to BCS were reviewed to confirm the location of clips, clip migration, the presence of complications such as hemorrhage or infection, and the effect of clips on treatment assessment. The location of a clip was categorized as either "within the tumor" or "outside the tumor." Clip migration was defined as the clip being located outside the proven malignancy at a distance of more than 1 cm. The signal void or artifact from inserted clips was reviewed for all signals of the breast MRI to evaluate negative effects on the assessment. Finally, we reviewed the total cost of the surgical clips compared to commercial markers.
Table 1 shows the summarized results, including immunohistopathological results and the documented time intervals. Pathology revealed cases of invasive ductal carcinoma (IDC), ductal carcinoma in situ (DCIS), and invasive carcinoma of no special type. One patient had bilateral cancer consisting of right breast IDC and left breast DCIS. After NAC, the final pathologic types were as follows: 11 residual invasive carcinomas of no special type, two IDC, and three DCIS. Among these, five lesions in five patients were treated with modified radical mastectomy, while 11 lesions in 10 patients underwent BCS. Image-guided localization or skin marking was performed in six patients. There was no difficulty in the pathological evaluation of a specimen due to inserted surgical clips. The majority of patients presented with T1 tumors (n=7, 43.8%; T1b with 2, T1c with 5), and 37.5% (n=6) had T2 tumors except for three DCIS lesions (Tis, 18.8%). The positive rate of estrogen receptor, progesterone receptor, and HER2 status was 81.3% (n=13), 62.5% (n=10), and 25% (n=4), respectively.
The time interval from clip insertion to postprocedural mammography and surgery was 47.1±14.7 days (median, 50.0 days) and 128.6±34.4 days (median, 132.0 days), respectively. The mean period between clip insertion and preoperative follow-up imaging was 122.6±34.8 days (median, 122.5 days) for mammography, 121.3±36.8 days (median, 122.5 days) for US, and 121.6±36.6 days (median, 122.5 days) for MRI. These data are presented in Table 1. The mean number of inserted clips was 2.3±0.7 (median, 2.0; range, 1-4).
There was no mammographic evidence of clip migration during postprocedural follow-up, preoperative final follow-up, and in surgical specimens (Figure 2). Moreover, no complication related to the clip insertion was noted during the study period, and no patient complained of heat sensation or pain during the MRI examination. On US, the inserted clips appeared as linear hyperechoic structures with or without posterior shadowing (Figure 3) and on MRI as small signal voids due to paramagnetic or susceptibility properties of the clips (Figure 4). However, there was no difficulty in evaluating the treatment response to NAC using US and MRI.
NAC is considered a standard step in the treatment of locally advanced breast cancer in its early stages. NAC is used frequently as it leads to better surgical results by conserving the breast while lessening complications and improving cosmetic outcomes [1,5]. The assessment of tumor response to NAC as confirmed on US, mammography, and MRI is important even if pCR is accomplished, which is reported to be 32.9% [3]. In this study, there was no case of pCR after NAC, but most of the enrolled cases showed a partial response to NAC, and some only had the in situ component without invasive foci. Moreover, BCS was performed in 10 patients, and among these, six underwent surgery under image-guidance, while the five remaining patients underwent modified radical mastectomy.
The commercial breast marker is generally placed at a CNB-performed site because radiologists cannot anticipate the exact outcome of NAC. As NAC has become more common, breast marker placement has become routine, especially in cases that may need additional excision based on the pathological findings [8,9,10,11,12]. Traditionally, breast markers are left behind for benign biopsies; for malignant lesions, markers are excised along with the cancerous lesion. In this way, breast marker usage is considered standard practice in malignant lesions [7].
There have been many types of titanium-based commercial breast markers launched by different companies. Prices range from US $75.00 to US $200 per clip internationally. However, until recently, none of these clips had been cleared for use by the KFDA. Thus, it has been impossible in South Korea to follow guidelines that suggest inserting tissue markers in a proven malignancy prior to NAC. As an alternative, traditionally used, less expensive surgical clips have been proven to be made of safe materials such as titanium, and have been approved by the KFDA. These clips can remain in the patient after surgery without serious complications, except for rare cases of allergic reactions. Moreover, recent studies have also shown that accurate tumor bed localization done by placing a surgical clip in the wall of a seroma cavity can assist the planning of radiation therapy following BCS [13,14]. Surgical clips can be easily and safely inserted, and the approximate cost in South Korea is about US $10 per clip. This study was premised upon the hypothesis that surgical clips could replace commercial breast markers because of their safety features and low cost.
In our study, all clips inserted as tissue markers were removed with the primary breast cancers during surgery. Surgical clips were placed via a commercial coaxial guiding needle used in the CNB of the breast. This procedure was easy to perform, and clip insertion required less than 5 minutes, as the procedure is similar to that of CNB under US guidance. Since clip insertion is done with US-guided CNB, it is performed with real-time imaging surveillance and regarded as a relatively safe method with few reported complications or adverse events. Therefore, the insertion process itself is not considered an onerous duty by breast radiologists. A similar method of using surgical clips to replace commercial breast markers has been reported by Uematsu [15] and Lee et al. [16], but in that study, the surgical clip was inserted by using an automated gun. Our study is the first to insert surgical clips with a semiautomatic gun via a guiding needle. This method is superior to the automated gun method as there is no need for repeat needle insertion into the mass. Thus, there is no tissue injury due to repeat insertion, and there is less bleeding and a lower probability of tumor cell seeding. If we can perform on-site clipping immediately after CNB for either a benign or malignant lesion, both medical costs and procedure time can be lower than those of the two-step clipping procedure used in our study.
The migration of surgical clips and related complications can be a major limitation of surgical clip insertion. The low tissue resistance of fatty breasts may allow clips to easily migrate; however, clips are generally inserted into the center of the mass. Thus, the chance of clip migration should be lower because of the higher tissue resistance [17]. Despite the mean time period from clipping to surgery of approximately 4 months (range, 47-169 days), there were no cases of clip migration in our study as confirmed on all imaging modalities, including specimen mammography after surgery. Moreover, there was no case of complications related to clip insertion. The other advantage of clip insertion was that the clips allowed easy detection of breast cancer on mammography and it was helpful for surgeons to explain breast cancer to their patients.
Several previous studies have reported that radiopaque markers are useful for tumor localization as well as for the assessment of tumor response after NAC, without disturbing radiologic multimodality evaluation including MRI [2,6,12,18]. We were able to evaluate tumor response to NAC and confirm the clip location by using multimodality imaging studies; the clips were visualized as a radiopaque metal density on radio-graphy, and as a hyperechoic linear structure with or without posterior shadowing on US. While breast MRI has proved superior to mammography and US in assessing tumor response for pCR [5,12], metal clips can cause artifacts on MRI, depending on magnetic susceptibility, clip quality, size, shape, orientation, position, and used MRI parameters [12]. In our study, the surgical clips created a small signal void on MRI; however, the primary malignancy was easily visualized on MRI. Moreover, the clinician can reassure patients by showing them the conspicuous decrease in exact mass after NAC, along with the internal dense radiopaque clip on mammography.
This study has several limitations. First, it was a retrospective study, and only patients who had agreed to surgical clip insertion and had undergone mammography, US, and MRI for scheduled BCS after NAC were selected. Therefore, a selection bias may exist. Second, the number of subjects was limited at only 16, a number too small to provide a reliable overall generalization from the study results. Further studies are needed for continued assessment of this procedure. Third, the placement of a surgical clip via a coaxial guide needle is not yet a globally approved method.
We concluded from this small study that surgical clips may replace commercial tissue markers for tumor localization in breast cancer patients undergoing NAC without migration. Our results have shown that surgical clips are well tolerated and safe for the patient, easily visualized on imaging, do not interfere with treatment response, and are cost-effective.
Figures and Tables
Figure 1
Schematic diagram of the preoperative ultrasonography (US)-guided surgical clip insertion. (A) The coaxial guiding needle with an inner stylet and surgical clips. (B) Under US-guidance (blue), the coaxial guiding needle (white) is inserted into the the center of the breast cancer (pink), and one or two clips (black) are passed through. The inner stylet (light blue) is reinserted for pushing the clip.
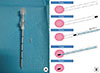
Figure 2
Mammography of a 40-year-old woman who underwent ultrasonography-guided surgical clip insertion due to left breast cancer. (A) Postprocedural follow-up mammography was performed after clipping, and showed metal clips in the center of the proven malignant mass. (B) At preoperative final follow-up mammography, the clips were located in the proven malignant mass which had decreased in size. There was no evidence of clip migration or other complications. (C) Specimen mammography was performed immediately after surgery, and there were metal clips visualized in the proven malignant lesion without evidence of clip migration. The pathologic result was invasive carcinoma of no special type, and a clear tumor margin was observed.
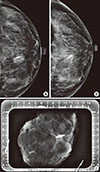
Figure 3
Preoperative ultrasonography (US)-guided surgical clip insertion. On US images, the coaxial needle (arrows) is visible as an echogenic white line and the clips show a linear hyperechoic structure (arrowhead) in the center of the proven malignancy.
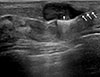
Figure 4
Magnetic resonance imaging (MRI) of a 40-year-old woman who underwent ultrasonography-guided surgical clip insertion due to left breast cancer. (A) There was a 19 mm-sized, fast, washout-enhancing malignancy at initial T1-weighted enhanced MRI with subtraction. (B) At preoperative follow-up MRI after neoadjuvant chemotherapy, a small signal void due to the clips (arrowhead) was observed in the center of the proven malignancy, which was much decreased in size and enhancement. However there was no difficulty in evaluating treatment response.
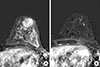
Table 1
Summarization of time intervals, operation, and immunohistopathological results for all patients

IDC=invasive ductal carcinoma; NST=invasive carcinoma of no special type; DCIS=ductal carcinoma in situ; MG1=postprocedural mammography; MG2=preoperative mammography; US=ultrasonography; MRI=magnetic resonance imaging; BCS=breast-conserving surgery; MRM=modified radical mastectomy; MG=mammography; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; (e)=equivocal.
References
1. Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007; 18:1927–1934.

2. Oh JL, Nguyen G, Whitman GJ, Hunt KK, Yu TK, Woodward WA, et al. Placement of radiopaque clips for tumor localization in patients undergoing neoadjuvant chemotherapy and breast conservation therapy. Cancer. 2007; 110:2420–2427.

3. Abdel-Razeq H, Marei L. Current neoadjuvant treatment options for HER2-positive breast cancer. Biologics. 2011; 5:87–94.
4. Kim Z, Min SY, Yoon CS, Lee HJ, Lee JS, Youn HJ, et al. The basic facts of Korean breast cancer in 2011: results of a nationwide survey and breast cancer registry database. J Breast Cancer. 2014; 17:99–106.

5. Lobbes MB, Prevos R, Smidt M, Tjan-Heijnen VC, van Goethem M, Schipper R, et al. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic review. Insights Imaging. 2013; 4:163–175.

6. Dash N, Chafin SH, Johnson RR, Contractor FM. Usefulness of tissue marker clips in patients undergoing neoadjuvant chemotherapy for breast cancer. AJR Am J Roentgenol. 1999; 173:911–917.

7. Kaufmann M, von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012; 19:1508–1516.

8. Margolin FR, Jacobs RP, Denny SR, Schrumpf JD. Clip placement after sonographically guided percutaneous breast biopsy. Breast J. 2003; 9:226–230.

9. Kopans DB. Clip placement during sonographically guided breast biopsy. AJR Am J Roentgenol. 2001; 176:1076–1077.

10. Guenin MA. Clip placement during sonographically guided large-core breast biopsy for mammographic-sonographic correlation. AJR Am J Roentgenol. 2000; 175:1053–1055.

11. Phillips SW, Gabriel H, Comstock CE, Venta LA. Sonographically guided metallic clip placement after core needle biopsy of the breast. AJR Am J Roentgenol. 2000; 175:1353–1355.

12. Genson CC, Blane CE, Helvie MA, Waits SA, Chenevert TL. Effects on breast MRI of artifacts caused by metallic tissue marker clips. AJR Am J Roentgenol. 2007; 188:372–376.

13. Tamai K, Mitsumori M, Fujishiro S, Kokubo M, Ooya N, Nagata Y, et al. A case of allergic reaction to surgical metal clips inserted for postoperative boost irradiation in a patient undergoing breast-conserving therapy. Breast Cancer. 2001; 8:90–92.

14. Coles CE, Wilson CB, Cumming J, Benson JR, Forouhi P, Wilkinson JS, et al. Titanium clip placement to allow accurate tumour bed localization following breast conserving surgery: audit on behalf of the IMPORT Trial Management Group. Eur J Surg Oncol. 2009; 35:578–582.

15. Uematsu T. Commercially available titanium clip placement following a sonographically guided core needle biopsy of the breast. Breast J. 2007; 13:624–626.

16. Lee SY, Kook SH, Kwag HJ. The results and usefulness of marker clip placement after ultrasound-guided mammotome excision of breast lesion. J Korean Radiol Soc. 2005; 52:207–213.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download