Abstract
Metaplastic breast cancer (MpBC) is an extremely rare breast cancer subtype, characterized by a heterogeneous phenotype. MpBC aggressive biology is attributed to its stem cell-like characteristics. Since these tumors are largely chemoresistant, novel targeted therapies should be explored. Herein, we report the clinical course of a 59-year-old African American woman with MpBC with a PIK3CA mutation in codon 545, exon 10 (GAG to AAG; p.Glu545Lys) and a TP53 mutation in codon 286, exon 8 (GAA to AAA; p.Glu286Lys). The same mutations were observed in the primary and secondary sites. The patient was treated with a molecularly matched therapy using a combined antiangiogenic and mammalian target of rapamycin kinase inhibitor strategy that included liposomal doxorubicin, bevacizumab, and temsirolimus. Partial remission was achieved. In this report, the scientific rationale underlying the activity of this combination was explored. In conclusion, patients may benefit from being offered molecular profiling early during the course of the disease to receive a therapy guided accordingly.
Metaplastic breast cancer (MpBC) is an extremely rare breast cancer subtype constituting 0.25% to 1% of all breast cancers [1]. Histopathologically, MpBC is characterized by a heterogeneous phenotype, most commonly with epithelial and mesenchymal components. MpBC is typically associated with rapid metastasis and poor survival compared to other breast cancer subtypes [1]. Most MpBCs are negative for estrogen receptor (ER), progesterone receptor (PR), and HER2/neu, and are managed as triple-negative breast cancer. MpBC also presents a poor response to conventional systemic therapy [1,2,3].
The pathogenesis of MpBC is not well understood. It has been suggested that the poor prognosis of MpBC could be due to its stem cell-like characteristics [3,4]. Phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit α (PIK3CA) mutations and loss of the phosphatase and tensin gene (PTEN) have been identified in this group, but their prognostic and therapeutic significance is unclear [3,5]. Due to its rarity, no clinical trial has been conducted specifically enrolling patients with MpBC. Tumor protein 53 (TP53) mutation and overexpression are present in 20% to 40% of breast cancers, but the incidence and significance of this mutation in MpBC are unknown [6].
Herein, we report the clinical course of a 59-year-old African American woman with MpBC presenting with PIK3CA and TP53 mutations, who was treated using a molecularly matched therapy.
A 59-year-old African American woman presented with a left-sided breast mass. The biopsy revealed a triple-negative, poorly differentiated, high-grade invasive ductal carcinoma with necrosis. Clinical staging indicated that the lesion was a T2 stage, N1 or 3, and M0 with biopsy-proven axillary metastasis and suspicion of internal mammary nodal involvement. The patient received standard neoadjuvant therapy with weekly paclitaxel (12 cycles) and 5-fluorouracil, adriamycin, and cyclophosphamide (FAC) (2 cycles). FAC was stopped due to poor tolerance, including febrile neutropenia and diarrhea. Subsequently, the patient declined further chemotherapy and underwent a left modified radical mastectomy followed by chest wall radiation.
The surgical pathological report indicated an invasive matrix-producing (metaplastic) carcinoma with patchy necrosis and 10% ER positivity, PR and HER2/neu negative, and stage pT2 N1 with 2 of 25 axillary lymph nodes presenting metastatic carcinoma. After radiation, the patient started a course of anastrozole, which was later switched to exemestane due to arthralgia. A year after surgery, the patient presented with pulmonary metastases and metastatic lymph nodes in the mediastinum and left internal mammary region (Figure 1).
The patient's tumor, including the left breast primary and the left axillary lymph node metastases were profiled separately. Genomic DNA polymerase chain reaction-based sequencing, using a next-generation sequencing (NGS) platform, was performed to screen for the frequently reported point mutations in a 46-gene panel on the primary and metastatic tissues. This 46-gene panel was validated using an NGS platform for the detection of frequently reported point mutations in human malignancies in the clinical laboratory improvement amendments-certified molecular diagnostics laboratory at The University of Texas MD Anderson Center. A minimum of 250×coverage is required at a given base for the interpretation of a wild-type or variant call. Although the NGS platform is capable of achieving a much higher analytical sensitivity, for clinical purposes, we determined the effective lower limit of detection of this assay (analytical sensitivity) for single-nucleotide variations to be in the range of 5% (one mutant allele in the background of 19 wild-type alleles) to 10% (one mutant allele in the background of nine wild-type alleles) by taking into consideration the depth of coverage at a given base and the ability to confirm low-level mutations using independent conventional platforms. To avoid false negatives, we required that the tumor nuclei represented 20% of the nuclei in the tested sample.
A PIK3CA gene mutation was detected in codon 545, exon 10 (GAG to AAG; p.Glu545Lys) and another mutation was found in theTP53 gene in codon 286, exon 8 (GAA to AAA; p.Glu286Lys). The same mutations were observed in the primary and the metastatic specimens.
The patient was given a combination therapy of intravenous temsirolimus, 25 mg weekly, plus intravenous bevacizumab, 15 mg/kg, and liposomal doxorubicin, 20 mg/m2, once on day 1 every 21 days [5]. The patient tolerated the regimen reasonably well with the exception of grade 1 fatigue. Repeat positron emission tomography/computed tomography (PET/CT) after two cycles of therapy showed a decrease in the standard uptake value from 12.3 to 3.9 in one intrathoracic lymph node and a decrease from 13.1 to 7.0 in another lesion as well as the complete resolution of the disease in most of the sites on a PET scan, with corresponding anatomic improvement observed on a CT scan (Figures 1,2,3). On the basis of the response, the therapy was pursued. PET/CT or CT-Chest/Abdomen/Pelvis was performed every 2 months to evaluate the response. After seven cycles, the patient requested a month break from chemotherapy related to grade 2-3 fatigue. The patient resumed her therapy with reasonable tolerance and continued with clinical benefit and radiological response. After 14 cycles of therapy, a plateau of response was reached and the patient developed grade 4 dyspnea. The scans indicated the progression of the disease and the therapy was discontinued.
The rarity, heterogeneity, and clinically aggressive nature of MpBC have made this disease a therapeutic challenge. Since these tumors are largely chemoresistant, the identification of novel targeted therapies is necessary to treat patients with this malignancy [5,6,7]. Early identification of targetable molecular aberrations, during the course of the disease, might provide therapeutic benefits, particularly in the absence of other options. In this patient, the combination of liposomal doxorubicin, bevacizumab, and temsirolimus was selected based on the strong scientific rationale that the PIK3CA mutation is found in approximately 14% to 21% of breast cancer patients referred to early phase clinical trials [8,9]. A phase I trial using this combination resulted in a 42% partial response (PR)/complete response (CR) rate in MpBC (5 out of 12 patients) [5]. Moreover, 39% of patients with a PIK3CA mutation and/or a PTEN mutation/loss (11 out of 28 patients) achieved a PR/CR [5]. In addition, an MpBC case series demonstrated the clinical benefits of this regimen. One patient presented a complete response [3].
Previous studies have demonstrated that therapies using mammalian target of rapamycin (mTOR) inhibitors show a response rate of 35% to 39% in patients presenting with activating mutations of the phosphatidylinositide 3-kinase/AKT/mTOR (PI3K/AKT/mTOR) pathway when compared to patients who did not have a documented PIK3CA mutation (response rate, 6%-10%) [8,9]. In addition to blocking the mTOR pathway, temsirolimus decreases hypoxia-inducible factor α (HIF-α) levels [10]. It has been shown that resistance to doxorubicin-based therapy is mediated through HIF-1α. Thus, HIF-1α inhibition by temsirolimus might increase the sensitivity to doxorubicin [11]. In addition to this putative synergy, the P53 tumor suppressor gene plays an important role in angiogenesis, apoptosis, and tumor stability. TP53 mutations are found in approximately 53% of patients referred to early phase clinical trials [12]. Intriguingly, TP53mut patients present a longer progression-free survival when treated with bevacizumab in combination with other drugs compared to patients treated without bevacizumab (10.97 months vs. 4.37 months) [12]. mTOR inhibitors can reduce vascular endothelial growth factor (VEGF) levels, increasing the antiangiogenic effects of bevacizumab [13]. The fact that MpBC commonly displays VEGF and HIF-α aberrations strengthens the rationale for using this combination [3,4]. A clinical trial combining these three agents for MpBC is ongoing and may help us to further define the effect of this combination.
In summary, identifying the underlying molecular aberrations in each patient as early as possible is crucial to select the most rational matched treatment regimen. Interestingly, in our case study, the same molecular aberrations were identified in both the primary and metastatic specimens. In addition to PIK3CA mutations, other mutations and gene amplifications have been reported in MpBC. Leibl and Moinfar [14] reported epidermal growth factor receptor amplification in 14 out of 20 patients with MpBC. Loss of PTEN was also reported by Moroney et al. [5]. The therapeutic implications of these mutations are unclear and further studies are warranted.
The case described in this study is unique in ways other than its specific molecular aberrations. The patient was initially diagnosed with triple-negative intraductal carcinoma of the breast. Later analysis of the surgical sample identified a matrix-producing (metaplastic) carcinoma with patchy necrosis and 10% ER positivity, although ER was not detected initially. These changes might be due to the heterogeneous nature of MpBC. Another important observation in this patient was the complete resolution of fluorodeoxyglucose (FDG) uptake in most lesions after treatment. Using mTOR inhibitors can lead to decreased metabolism in cancer cells due to their predominant cytostatic properties. FDG uptake, which is reflective of metabolic activity, may be a useful biomarker to monitor the response to mTOR inhibitor treatments. Additionally, PET/CT might be a better imaging modality than CT alone to evaluate the tumor response in patients treated with mTOR inhibitors [15].
In conclusion, MpBC is a heterogeneous breast cancer with no current standard therapy. Patients may benefit from being offered molecular profiling early during the course of the disease followed by a therapy guided accordingly. Liposomal doxorubicin+bevacizumab+temsirolimus is one combination that has a strong underlying scientific rationale, and could be used a valuable therapeutic strategy for patients with MpBC.
ACKNOWLEDGMENTS
The authors acknowledge Joann Aaron, MA, in the Department of Investigational Cancer Therapeutics at MD Anderson for editorial assistance.
Notes
References
1. Pezzi CM, Patel-Parekh L, Cole K, Franko J, Klimberg VS, Bland K. Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol. 2007; 14:166–173. PMID: 17066230.

2. Bae SY, Lee SK, Koo MY, Hur SM, Choi MY, Cho DH, et al. The prognoses of metaplastic breast cancer patients compared to those of triple-negative breast cancer patients. Breast Cancer Res Treat. 2011; 126:471–478. PMID: 21287362.

3. Moulder S, Moroney J, Helgason T, Wheler J, Booser D, Albarracin C, et al. Responses to liposomal Doxorubicin, bevacizumab, and temsirolimus in metaplastic carcinoma of the breast: biologic rationale and implications for stem-cell research in breast cancer. J Clin Oncol. 2011; 29:e572–e575. PMID: 21482991.

4. Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009; 69:4116–4124. PMID: 19435916.

5. Moroney J, Fu S, Moulder S, Falchook G, Helgason T, Levenback C, et al. Phase I study of the antiangiogenic antibody bevacizumab and the mTOR/hypoxia-inducible factor inhibitor temsirolimus combined with liposomal doxorubicin: tolerance and biological activity. Clin Cancer Res. 2012; 18:5796–5805. PMID: 22927482.

6. Nagai MA, Schaer Barbosa H, Zago MA, Araújo Silva W Jr, Nishimoto IN, Salaorni S, et al. TP53 mutations in primary breast carcinomas from white and African-Brazilian patients. Int J Oncol. 2003; 23:189–196. PMID: 12792793.

7. Rayson D, Adjei AA, Suman VJ, Wold LE, Ingle JN. Metaplastic breast cancer: prognosis and response to systemic therapy. Ann Oncol. 1999; 10:413–419. PMID: 10370783.

8. Janku F, Wheler JJ, Westin SN, Moulder SL, Naing A, Tsimberidou AM, et al. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol. 2012; 30:777–782. PMID: 22271473.

9. Janku F, Tsimberidou AM, Garrido-Laguna I, Wang X, Luthra R, Hong DS, et al. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther. 2011; 10:558–565. PMID: 21216929.

10. Pantuck AJ, Zeng G, Belldegrun AS, Figlin RA. Pathobiology, prognosis, and targeted therapy for renal cell carcinoma: exploiting the hypoxia-induced pathway. Clin Cancer Res. 2003; 9:4641–4652. PMID: 14581333.
11. He C, Sun XP, Qiao H, Jiang X, Wang D, Jin X, et al. Downregulating hypoxia-inducible factor-2α improves the efficacy of doxorubicin in the treatment of hepatocellular carcinoma. Cancer Sci. 2012; 103:528–534. PMID: 22145922.

12. Said R, Hong DS, Wheler JJ, Naing A, Falchook GS, Fu S, et al. p53 mutations in advanced cancers: clinical characteristics and outcomes in a phase I setting. J Clin Oncol. 2012; 30:Abstract #10607.

13. Del Bufalo D, Ciuffreda L, Trisciuoglio D, Desideri M, Cognetti F, Zupi G, et al. Antiangiogenic potential of the mammalian target of rapamycin inhibitor temsirolimus. Cancer Res. 2006; 66:5549–5554. PMID: 16740688.

14. Leibl S, Moinfar F. Metaplastic breast carcinomas are negative for Her-2 but frequently express EGFR (Her-1): potential relevance to adjuvant treatment with EGFR tyrosine kinase inhibitors? J Clin Pathol. 2005; 58:700–704. PMID: 15976335.

15. Cejka D, Kuntner C, Preusser M, Fritzer-Szekeres M, Fueger BJ, Strommer S, et al. FDG uptake is a surrogate marker for defining the optimal biological dose of the mTOR inhibitor everolimus in vivo. Br J Cancer. 2009; 100:1739–1745. PMID: 19436299.

Figure 1
Pretreatment fluorodeoxyglucose positron emission tomography/computed tomography of the patient showing multiple hypermetabolic pulmonary metastasis and hypermetabolic adenopathy in the thorax.
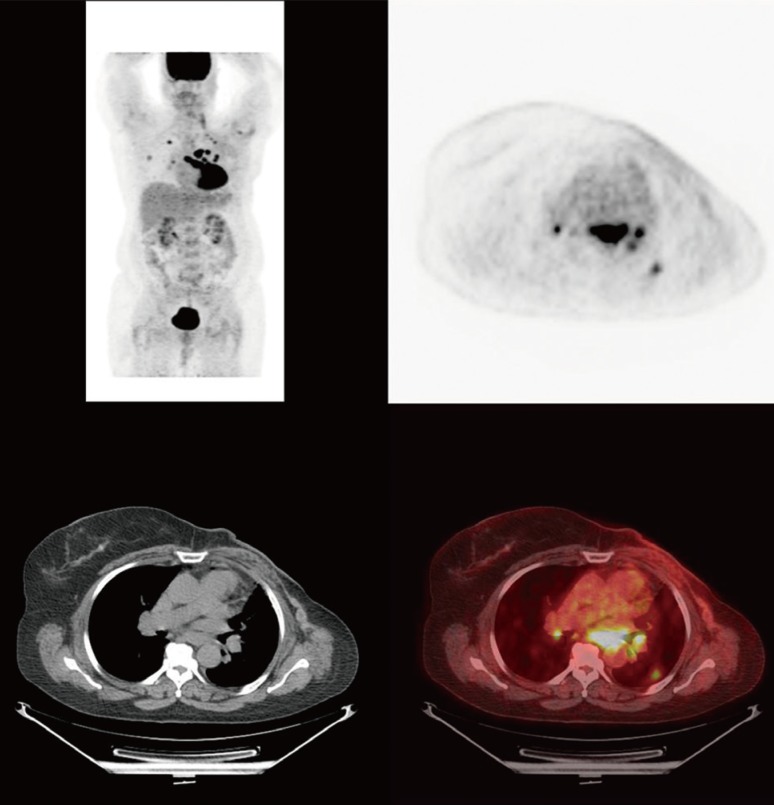
Figure 2
Positive positron emission tomography/computed tomography response after two cycles of combination therapy with liposomal doxorubicin, bevacizumab, and temsirolimus.
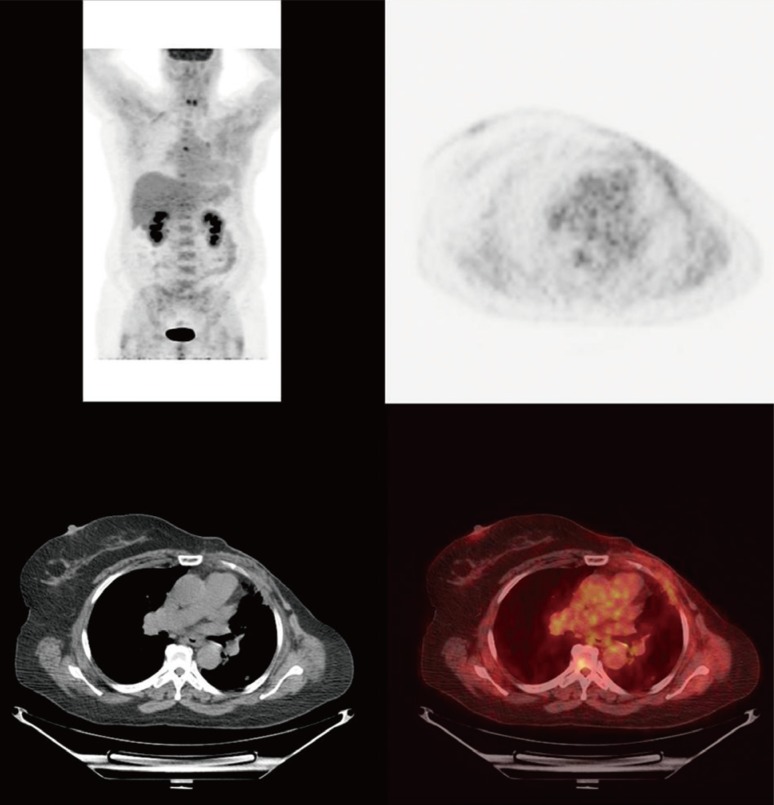
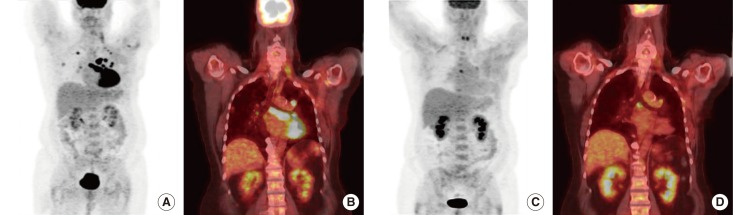
Figure 3
Pretreatment and posttreatment maximum intensity projection image and coronal images of the patient. Pretreatment maximum intensity projection image and coronal image showing hypermetabolic pulmonary metastasis and lymphadenopathy (A, B). Posttreatment maximum intensity projection image and coronal image showing response to therapy after two cycles of combination of liposomal doxorubicin, bevacizumab, and temsirolimus (C, D).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download