Abstract
Purpose
Preoperative chemotherapy has been used to increase the rate of breast conserving surgery (BCS) in Caucasian women. However, whether it would also increase the rate of BCS in Korean women has not been verified. The aim of this study was to determine the effectiveness of preoperative chemotherapy to make BCS possible in Korean women who have locally advanced cancer without any increase of locoregional recurrence according to operation methods (BCS vs. mastectomy).
Methods
From August 2002 to April 2005, 205 patients with stage II or III breast cancer were enrolled in a phase III randomized trial of preoperative chemotherapy. Surgeons decided on the type of surgery (mastectomy or BCS) at initial diagnosis. By randomization, patients received four cycles of either docetaxel/capecitabine or doxorubicin/cyclophosphamide followed by surgery and crossover to the other treatment as postoperative chemotherapy.
Results
The mean tumor size was 3.29 cm and the mean breast volume was 489 cc at diagnosis. After preoperative chemotherapy, clinical response was shown in 76.0% of the patients. Of the 71 patients planned for a mastectomy at initial diagnosis, 27 patients underwent BCS (38.0%). Clinical T stage after preoperative chemotherapy, pathologic T size and lymphatic invasion were correlated with conversion to BCS. In multivariate analysis, only lymphatic invasion showed statistical significance. Locoregional disease-free survival did not statistically differ between the two operation methods for the patients who were planned for a mastectomy at the initial exam.
Preoperative chemotherapy has become one of the treatment options for patients with locally advanced breast cancer [1,2]. The National Surgical Adjuvant Breast Project (NSABP) protocol B-18 was a large, prospectively randomized clinical trial designed to compare the effects of doxorubicin and cyclophosphamide (AC) administered either preoperatively or in a more traditional postoperative regimen. The results of this trial showed that more patients could undergo lumpectomy as primary surgical treatment than was initially considered feasible, particularly in those patients whose tumors clinically measured ≥5.1 cm [3,4].
Breast cancer in Korea continues to rise each year, and it has become the most common cancer in Korean women since 2001. Breast cancer patients in Korea also have a strong preference for breast conserving surgery (BCS). The rate of BCS has been increasing each year. However, the rate is relatively lower in Korea than in European countries [5]. Preoperative chemotherapy has been used to increase the rate of BCS in Caucasian women [3,4]. However, whether it would also increase the rate of BCS in Korean women has not been verified.
We initiated a phase III randomized study of preoperative chemotherapy with AC versus docetaxel/capecitabine (TX) for stage II and III breast cancer [6]. We report that this study demonstrated that the combination of docetaxel plus capecitabine was effective and well tolerated and compares favorably to AC as preoperative chemotherapy for the treatment of early breast cancer. TX showed a trend toward increased pCR in patients with poor prognostic factors and hormone receptor-positive tumors [6].
Therefore, the aim of this study was to determine the effectiveness of preoperative chemotherapy to make BCS possible in Korean women who have locally advanced cancer, while avoiding any increase of locoregional recurrence according to operation methods (BCS vs. mastectomy).
In June 2002, we initiated a phase III randomized study of preoperative chemotherapy with AC versus TX for stage II and III breast cancer [6]. The study was approved by an Independent Review Board at the National Cancer Center of Korea in accordance with local regulations and was conducted in accordance with the Declaration of Helsinki. All participating patients provided us with informed consent.
We used a stratified block randomization of block size four according to disease status (stage II or III), estrogen receptor status (positive or negative), and age (<50 or ≥50 years).
The study profile is shown in Figure 1. All patients needed to have histologically confirmed and newly diagnosed stage II and III breast cancer. We used fluorine-18 fluorodeoxyglucose positron emission tomography (18F-FDG-PET) or fine needle aspiration biopsy (FNAB) to determine a positive axillary lymph node on initial examination. The patients who had received hormonal therapy, chemotherapy or radiotherapy for breast cancer were previously excluded in this trial. The patients who underwent breast operation other than biopsy for diagnosis were also excluded. From June 2002 to March 2005, 205 patients were enrolled in this trial.
The patients underwent preoperative chemotherapy, operation and postoperative chemotherapy. The surgery consisted of either modified radical mastectomy or BCS with axillary lymph node dissection. The surgeon decided which type of surgery was adequate for each patient at initial diagnosis. Mastectomy was decided at initial diagnosis if the patients had a large tumor in a small breast, multiple tumors in different quadrants or diffusely scattered suspicious microcalcification on the breast. Subsequently, patients underwent the preoperative chemotherapy according to randomization. For 101 patients, doxorubicin (60 mg/m2)/cyclophosphamide (600 mg/m2) was administered intravenously on day 1 of each 3-week cycle. For 103 patients, docetaxel 36 mg/m2 was administered as intravenous infusion on day 1 of each 3-week cycle along with capecitabine 1,000 mg/m2 p.o. b.i.d. on days 1-14. The cycle was repeated 4 times, every 3 weeks. If the patients refused the chemotherapy or received chemotherapy with dose-reduction, we excluded these patients in this study. Operation followed within 3 weeks after the fourth cycle of the preoperative chemotherapy. If patients showed closed margin status (<2 mm) in the pathologic report, the surgeons performed further excision on the patients. Postoperative chemotherapy was given as follows: AC for 4 cycles for the patients who received TX before surgery and TX for 4 cycles for the patients who received AC before surgery. After completion of postoperative chemotherapy, all patients received radiotherapy and concurrently endocrine therapy with tamoxifen or anastrozole when the hormonal receptor was positive. The patients who clinically showed lymph node metastasis also received radiotherapy, although they underwent modified radical mastectomy.
Clinical response was assessed using the response evaluation criteria in solid tumors (RECIST) criteria [7]. Baseline examinations were performed prior to the first cycle of chemotherapy and included a physical examination, PET with 18-fluorodeoxyglucose (FDG-PET) or PET combined with computed tomography (CT) to exclude node-negative and stage IV disease, and ultrasound to determine tumor size as described in the previously published paper [6]. All the focal FDG accumulations for the ipsilateral, contralateral axillary, internal mammary, supraclavicular and infraclavicular nodal groups and for any extranodal foci were identified. Focal FDG accumulations above 1 mL/g in standardized uptake value, which could not be explained by overt inflammation, were diagnosed positive for metastases [8-10]. Ultrasound of the affected breast was repeated prior to the third cycle of chemotherapy and surgery to evaluate tumor and lymph node size. Partial and complete responses were confirmed with repeat imaging as described in the previously published paper [6]. Clinical stage was determined according to the 6th edition of American Joint Committee on Cancer (AJCC). Breast volume was calculated by CT in 100 patients available before preoperative chemotherapy, and was calculated by the summation of segmented monolayers. Locoregional recurrence is defined if the patients showed the recurrence on the ipsilateral breast and axillary lymph node.
To determine the correlating factors to BCS, univariate comparisons of the study variables were assessed by chi-square tests for categorical variables and t-test for continuous variables. Multivariate analysis was also performed to estimate correlating factors with conversion to BCS in patients who were planned for mastectomy at initial diagnosis. Kaplan-Meier curves were used to calculate locoregional disease-free survival. For an operation method group comparison, a log-rank test was used in the survival analysis. All statistical analyses were performed with STATA version 10.0 (StataCorp LP, College Station, USA).
The median age was 44.0 years (range, 21-67 years), and the mean tumor size was 3.29 cm (range, 0.9-12 cm) at initial diagnosis and 1.71 cm (range, 0.0-8.0 cm) after 4 cycles of preoperative chemotherapy. The mean reduction of tumor size was 1.58 cm. The mean breast volume was 489 cc. At initial diagnosis, the surgeon planned mastectomy in 71 patients (34.8%). After 4 cycles of CTx, the surgeon performed mastectomy in 61 patients (29.9%) and BCS in 143 patients (70.1%). After 4 cycles of preoperative chemotherapy, clinical response was shown in 76.0% (CR, 4.4%; PR, 71.6%) by the RECIST criteria. Tables 1 and 2 show the patients' characteristics and clinical tumor response in this study.
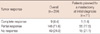
The surgeons decided the operation method for the enrolled patients at initial diagnosis. They planned mastectomy for 71 patients at initial diagnosis. After 4 cycles of preoperative chemotherapy, they performed BCS in 27 patients among the 71 patients with a planned mastectomy at initial diagnosis. The conversion rate was estimated at 38.0% (Table 3).
In all 204 patients, T stage, tumor size, N stage, and TNM stage at initial diagnosis were the factors correlated with BCS on univariate analysis. Microcalcification on mammography, multicentricity of tumor, tumor size, T stage, N stage, TNM stage in pathologic finding and lymphatic invasion were also correlating factors with BCS on univariate analysis (Table 4). However, multivariate analysis showed that multicentricity, T stage and N stage in the pathologic finding were significantly correlated with BCS (Table 5).
In 71 patients with a planned mastectomy at initial diagnosis, T stage at initial diagnosis, lymphatic invasion, tumor size and TNM stage in the pathologic finding were correlated with conversion to BCS after preoperative chemotherapy on univariate analysis (Table 4). Among these, lymphatic invasion was only significantly correlated with conversion to BCS on multivariate analysis (Table 6).
The mean follow-up time was 52.3 months (range, 5-74 months). Locoregional disease-free survival did not statistically differ between the two groups (performed BCS vs. performed mastectomy) in the patients with a planned mastectomy at initial exam (p=0.649).
In this section, we analyze the effectiveness of preoperative chemotherapy to make BCS possible in Korean women. A large number of consecutive patients who had locally advanced breast cancer and planned preoperative chemotherapy and operation were enrolled in this prospectively designed, single-institute study. This study differs from other studies in that our study might be the first efficiently designed trial about the effectiveness of preoperative chemotherapy to make BCS possible in Korean women who have advanced cancer. We decided which type of surgery was adequate for the patients at initial diagnosis. Subsequently, patients underwent the preoperative chemotherapy according to randomization and operation. Twenty-seven patients among 71 patients (38%) who could not avoid mastectomy at initial diagnosis underwent BCS after preoperative chemotherapy. Although 17 patients could undergo BCS at initial diagnosis, they sought mastectomy after preoperative chemotherapy.
In a number of important randomized trials (National Surgical Adjuvant Breast and Bowel Project [NSABP] B-18, NSABP B-27, Mauriac et al., Scholl et al., European Organization for Research and Treatment of Cancer [EORTC] 10902, Petrov Institute, Powles et al.) patients with breast cancer were evaluated and chemotherapy was compared in the preoperative or the postoperative setting [3,11-18]. From these trials, the effectiveness of preoperative chemotherapy on operation method was also reported. The NSABP B-18 trial was the first study to compare postoperative with preoperative chemotherapy. The NSABP protocol B-18 reported that the size of primary breast tumors was clinically reduced in 80% of patients treated preoperatively and no tumor could be detected clinically in 36% of the patients [3,4]. The trial resulted in a 7% higher rate of BCS compared with the postoperative treatment arm (60% vs. 67%, p<0.01) [3]. The European Cooperative Trial in operable breast cancer (ECTO) showed an increase in BCS from 34% in the postoperative treatment arm to up to 65% by preoperative chemotherapy (p<0.001) [19]. The EORTC 10902 trial also demonstrated an improvement in the BCS rate by preoperative chemotherapy: 21% (postoperative treatment arm) vs. 37% (preoperative treatment arm) [16]. Moreover, the EORTC 10902 trial showed that 23% underwent BCS among the patients who were planned for mastectomy in the preoperative chemotherapy group [16]. Our study showed that the conversion rate to BCS was 38%. This result translates into a successful effect of preoperative chemotherapy in this study compared with other studies in Westernized countries. A few studies have been reported on BCS after preoperative chemotherapy for Asian patients [20,21]. However, in these studies, relatively small numbers of patients (30 patients) were enrolled compared with our study (204 patients). We also enrolled stage IIA patients in this study, although all of these were patients with T1N1M0.
Another study has reported three preoperative factors that are the main predictors for breast conservation: the existence of microcalcification as seen in the pretreatment mammography, the postchemotherapy tumor size, and the type of mammographic lesions [22]. Clinical tumor sizes, nodal status and clinical CR were demonstrated as the correlating factors with BCS in the NASBP B-18 trial [3]. We showed that multicentricity, T stage and N stage in the pathologic finding were significantly correlated with BCS performed in all 204 patients (Table 5). The presence of tumor multicentricity may be correlated with the possibility of a diffuse tumor extent and the preoperative chemotherapy may not be effective in this case. Advanced T stage in the pathologic finding can also translate into a poor response to preoperative chemotherapy and the tumor may not shrink effectively with preoperative chemotherapy. However, Table 6 shows that lymphatic invasion was only the significant correlating factor with conversion to BCS in patients who were appropriate for mastectomy at initial diagnosis. However, the reason why the lymphatic invasion is only one factor correlating with conversion is not clear.
We planned mastectomy at the initial exam for the patients who showed multifocal tumor and diffuse suspicious microcalcification. After preoperative chemotherapy, we could not change the mastectomy into breast conversion surgery in these patients. The preoperative chemotherapy may not be effective on these cases. Seven patients showed a closed margin or positive margin in the pathologic report. They underwent re-excision to obtain a safe margin status. However, we did not mastectomy in these patients.
We used an ultrasonogram to measure the size of the tumor before preoperative chemotherapy and after preoperative chemotherapy in this study. We also used the ultrasonogram to measure the size in the patients who showed a multifocal tumor. However, in their mammography, some patients showed diffuse suspicious microcalcification with a mass. We also used mammography to measure the size of the tumor in a number of these patients.
We found no significant difference in locoregional disease-free survival according to operation method after preoperative chemotherapy in the patients with a planned mastectomy at the initial exam. This result is similar to the previously reported studies in which patients underwent surgery followed by postoperative chemotherapy [23-25]. In a preoperative chemotherapy setting, some studies showed a higher local recurrence rate in the mastectomy group than in BCS group. Veronesi et al. [26] reported that the local recurrence rate was 5.9% and 21.7% in the BCS and mastectomy groups, respectively. McIntosh et al. [27] also reported that the local recurrence rate was 2% and 7% in the BCS and mastectomy groups, respectively. However, Touboul et al. [28] showed no significant difference in local recurrence survival between the BCS and mastectomy groups after preoperative chemotherapy. Surgeons have been concerned that down-staged tumor by preoperative chemotherapy might result in satellite nodules rather than concentric shrinkage and they may prefer mastectomy to BCS for locally advanced patients. However, no significant difference was shown in locoregional recurrence in our study. The NSABP B-18 study showed no statistically significant differences in disease-free survival and overall survival between the preoperative chemotherapy group and the postoperative chemotherapy group after 16 years follow-up [29].
We attempted to calculate breast volume by CT before preoperative chemotherapy. This was calculated by the summation of segmented monolayers. Because the qualities of image data were poor in some patients, we could only calculate breast volume in 100 patients.
In this study, preoperative chemotherapy would also be effective to raise the rate of BCS in Korean women who have locally advanced breast cancer. However, locoregional disease-free survival did not statistically differ between BCS and mastectomy after preoperative chemotherapy. Therefore, more opportunity may be considered to undergo BCS in Korean patients who have locally advanced cancer by preoperative chemotherapy.
Figures and Tables
Figure 1
Study profile.
TX=docetaxel+capecitabine; AC=doxorubicin+cyclophosphamide; RECIST=response evaluation criteria in solid tumors.
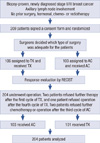
Table 3
Difference of operative methods between initial plan and performance after preoperative chemotherapy
ACKNOWLEDGEMENTS
Sanofi-Aventis and Roche Korea provided the study drugs, Taxotere and Xeloda, respectively.
Notes
This study was supported in part by the NCC Grant 0210150 and the Korean Health 21 R&D Project Grant by Ministry of Health and Welfare, Republic of Korea (0412-CR01-0704-0001).
References
1. Wolff AC, Davidson NE. Preoperative therapy in breast cancer: lessons from the treatment of locally advanced disease. Oncologist. 2002. 7:239–245.

2. Buzdar AU. Preoperative chemotherapy treatment of breast cancer: a review. Cancer. 2007. 110:2394–2407.

3. Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997. 15:2483–2493.

4. Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998. 16:2672–2685.

5. Ahn SH, Yoo KY. Korean Breast Cancer Society. Chronological changes of clinical characteristics in 31,115 new breast cancer patients among Koreans during 1996-2004. Breast Cancer Res Treat. 2006. 99:209–214.

6. Lee KS, Ro J, Nam BH, Lee ES, Kwon Y, Kwon HS, et al. A randomized phase-III trial of docetaxel/capecitabine versus doxorubicin/cyclophosphamide as primary chemotherapy for patients with stage II/III breast cancer. Breast Cancer Res Treat. 2008. 109:481–489.

7. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. European Organization for Research and Treatment of Cancer. National Cancer Institute of the United States. National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000. 92:205–216.

8. Inoue T, Yutani K, Taguchi T, Tamaki Y, Shiba E, Noguchi S. Preoperative evaluation of prognosis in breast cancer patients by [(18)F]2-Deoxy-2-fluoro-D-glucose-positron emission tomography. J Cancer Res Clin Oncol. 2004. 130:273–278.

9. Wahl RL, Siegel BA, Coleman RE, Gatsonis CG. PET Study Group. Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer: a report of the staging breast cancer with PET Study Group. J Clin Oncol. 2004. 22:277–285.

10. Zornoza G, Garcia-Velloso MJ, Sola J, Regueira FM, Pina L, Beorlegui C. 18F-FDG PET complemented with sentinel lymph node biopsy in the detection of axillary involvement in breast cancer. Eur J Surg Oncol. 2004. 30:15–19.

11. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001. (30):96–102.

12. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006. 24:2019–2027.

13. Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003. 21:4165–4174.

14. Mauriac L, MacGrogan G, Avril A, Durand M, Floquet A, Debled M, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonié Bordeaux Groupe Sein (IBBGS). Ann Oncol. 1999. 10:47–52.

15. Scholl SM, Fourquet A, Asselain B, Pierga JY, Vilcoq JR, Durand JC, et al. Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumours considered too large for breast conserving surgery: preliminary results of a randomised trial: S6. Eur J Cancer. 1994. 30A:645–652.

16. van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001. 19:4224–4237.

17. Semiglazov VF, Topuzov EE, Bavli JL, Moiseyenko VM, Ivanova OA, Seleznev IK, et al. Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage IIb-IIIa breast cancer. Ann Oncol. 1994. 5:591–595.

18. Powles TJ, Hickish TF, Makris A, Ashley SE, O'Brien ME, Tidy VA, et al. Randomized trial of chemoendocrine therapy started before or after surgery for treatment of primary breast cancer. J Clin Oncol. 1995. 13:547–552.

19. Gianni L, Baselga J, Eiermann W, Porta VG, Semiglazov V, Garcia-Conde J, et al. First report of the European Cooperative Trial in operable breast cancer (ECTO): effects of primary systemic therapy PST on local-regional disease. 2002. 21:In : 2002 American Society of Clinical Oncology Annual Meeting; Abstract #132.
20. Viswambharan JK, Kadambari D, Iyengar KR, Srinivasan K. Feasibility of breast conservation surgery in locally advanced breast cancer downstaged by neoadjuvant chemotherapy: a study in mastectomy specimens using simulation lumpectomy. Indian J Cancer. 2005. 42:30–34.

21. Zhou B, Yang DQ, Qiao XM, Tong FZ, Cao YM, Liu P, et al. Feasibility of breast conservation surgery after neoadjuvant chemotherapy for breast cancer. Zhonghua Yi Xue Za Zhi. 2005. 85:769–772.
22. Sadetzki S, Oberman B, Zipple D, Kaufman B, Rizel S, Novikov I, et al. Breast conservation after neoadjuvant chemotherapy. Ann Surg Oncol. 2005. 12:480–487.

23. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002. 347:1233–1241.

24. van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000. 92:1143–1150.

25. Jacobson JA, Danforth DN, Cowan KH, d'Angelo T, Steinberg SM, Pierce L, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995. 332:907–911.

26. Veronesi U, Bonadonna G, Zurrida S, Galimberti V, Greco M, Brambilla C, et al. Conservation surgery after primary chemotherapy in large carcinomas of the breast. Ann Surg. 1995. 222:612–618.

27. McIntosh SA, Ogston KN, Payne S, Miller ID, Sarkar TK, Hutcheon AW, et al. Local recurrence in patients with large and locally advanced breast cancer treated with primary chemotherapy. Am J Surg. 2003. 185:525–531.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download