INTRODUCTION
Breast cancer is the most common cancer and then leads to most of cancer deaths among women worldwide. A wide variety of chemotherapeutics are being explored to treat breast cancer. Anti-estrogen hormone therapy has been in best used for prevention and treatment in women with early breast cancer.(1) However, hormone therapy has little effect on estrogen receptor α (ERα)-negative tumors.(2) In deed, approximately 30% of breast cancer patients are negative for ERα expression at diagnosis. Furthermore, approximately 50% of patients with advanced disease do not respond to first-line treatment with hormone therapy.(3) Therefore, resistance to hormone therapy causes a major problem in treatment and prevention of breast cancer. Thus, in order to overcome resistance to hormone therapy the novel treatment strategies should be explored.
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated nuclear receptors that mediate transcriptional regulation of genes involved in the oxidation, transport, and storage of lipids.(4-7) Among the three PPAR isoforms (PPARα, β, and γ),(8-10) PPARγ influences such biological processes as inflammation, cell survival, differentiation, cell proliferation, and tumorigenesis.(11) Several ligands for PPARγ have been identified including endogenous 15-deoxy-Δ12,14-prostaglandin J2, linoleic acid, lysophophatidic acid, and the thiazolidinediones class of synthetic antidiabetic drugs such as troglitazone and rosiglitazone.(12-18)
In the other hand, PPARg ligand has recently been incriminated as a potential target for the prevention and treatment of human cancers.(17) In breast cancer, PPARγ ligands also inhibit proliferation and induce apoptosis in ERα-positive breast cancer cells.(19,20) These reports indicate that PPARγ ligand may prove to have a role in breast cancer treatment /prevention in the future. However, it has recently been known that ERα negatively interferes PPARγ signaling in breast cancer cells,(21) indicating that PPARγ ligand have not full activity for ERα-positive MCF-7 breast cancer cells. In deed, PPARγ activation by PPARγ ligand has little apparent clinical value among patients with treatment?refractory breast cancer.(22) Therefore, it is likely to suggest that PPARγ ligand may be more useful in ERα-negative MDA-MB-231 breast cancer cells compared to ERα-positive MCF-7 breast cancer cells. Moreover, effect of PPARγ ligand on cell proliferation of ERα-negative MDA-MB-231 breast cancer cells is not known.
Therefore, we attempted to elucidate the role of PPARγ in ERα-negative breast cancer carcinogenesis and explore the possibility of using PPARγ ligand as chemopreventive agent for hormone therapy-resistant breast cancer patients. In the current study, we determined if PPARγ ligand induces cell-cycle arrest and apoptosis in ERα-negative MDA-MB-231 breast cancer cell line. The observed apoptotic activity of PPARγ ligand was accompanied by a cell cycle regulator induction such as p21. This paper shows the first time evidences that PPARγ ligand plays as a primary member of chemotherapeutic candidates for ERα-negative breast cancer. So, we expect the clinical use of PPARγ ligand, which is more useful for in ERα-negative breast cancers compared to ERα-positive cancers.
METHODS
Materials
Anti-CDK (2,4,6), cyclin (A, D1, D2, E), p21, p27, p-Rb, and PPARγ were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Fetal bovine serum (FBS) and charcoal-dextran treated FBS were obtained from Gibco BRL (Life Technologies, Grand Island, USA). HBSS (Hanks balanced salt solution), MTT, propidium iodide, RPMI-1640, and β-actin antibody were obtained from Sigma Chemical Co. (St. Louis, USA). Ciglitazone, rosiglitazone, and troglitazone were purchased from ALEXIS Biochemicals (Lausen, Switzerland).
Cell culture
A human breast cancer cell line, MDA-MD-231 was obtained from the American Type Culture Collection (Rockville, USA). The cells were cultured in RPMI medium containing 10% fetal calf serum, 2 mM glutamine, antibiotics (Penicillin G 60 mg/L, Streptomycin 100 mg/L, Amphotericin B 50 ㎕/L) under a humid atmosphere (37 ℃, 5% CO2).
MTT assay
The effect of PPARγ ligands on cell viability of MDA-MB-231 cells was determined using MTT assay. Viability of cultured cells was determined by reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma, USA) to formazan. Briefly, cells of 1×104 cells/well were inoculated into a 96-well plate, treated with ciglitazone, rosiglitazone or troglitazone at various concentrations. After incubation for 72 h, cells were washed twice with phosphate-buffered saline (PBS), and MTT (100 µg/0.1 ml PBS) was added to each well. Cells were incubated at 37 ℃ for 1 h, and 100 ?l dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. The plate was read in a microplate reader (model 3550, BIO-RAD, Richmond, USA) at 570 nm.
Cell cycle analysis
For analysis of cell cycle, 5 × 105 cells were seeded onto 6-well plates and treated with troglitazone at various concentrations for 48 h. At indicated times, cells were harvested by trypsinization, centrifuged at 1500 rpm for 3 min, washed with PBS, and fixed 1hour in 70% ethanol at 4 ℃ (fixed with 70% ethanol for 1h at 4 ℃), and then collected by centrifugation, resuspended in PBS containing 5 µg/ml RNase and 50µg/ml propidium iodide (PI), and incubated at 4 ℃ for 1h, protected from light. DNA content was analyzed using Becton Dickinson FACScan and Cell Quest software. Subsequent data analysis was performed using ModFit software (Becton Dickinson United Kingdom Ltd., Cowley,
UK).
Western blot analysis
After washing with PBS and harvesting, cell pellets were lysed with the lysis buffer (50 mM Tris-HCl, pH 7.6, 1% Triton-X 100, 2 mM EDTA, 0.5% SDS, 150 mM NaCl, 1 mM sodium orthovanadate, 2 mM EGTA, 4 mM p-nitro-phenyl phosphate, and 100 mM sodium fluoride) supplemented with protease inhibitors (0.5% leupeptin, 0.5% aprotinin, and 0.02% phenylmethylslfonyl fluoride). After incubation for 30 min at 4 ℃, cellular debris was removed by centrifugation at 10,000×g for 30 minute and supernatants were analyzed by 12% SDS-PAGE. Electrophoretic transfer from slab gel to nitrocellulose paper and subsequent immunoblotting was performed by incubation with primary antibodies and followed by further incubation with HRP-conjugated secondary antibody. Reactive proteins were detected using enhanced chemiluminescence (ECL, Amersham Life Sciences, Arlington Heights, USA).
TUNEL staining
Detection of apoptosis in breast cancer cell was carried out using a DNA fragmentation assay based on terminal deoxynucleotidyl transferase (TdT)-mediated dUTP digoxigenin nick-end-labeling (TUNEL). Briefly, MDA-MB-231 breast cancer cells plated on glass coverslips in 24-well culture plates were grown at 37 ℃ for 24 h, troglitazone was added and incubated for an additional 48 h. The cells were then fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min, washed with PBS, and incubated in 0.3% H2O2 to block endogenous peroxidases and incubated with a TUNEL reaction mixture (terminal deoxynucleotidyl transferase, nucleotide mixture, Roche, Mannheim, Germany) at 37 ℃ for 1 h, and then the sections were washed with distilled water (D/W). They were then reincubated in anti-fluorescein antibody conjugated with horse-radish peroxidase at room temperature for 30 min, re-washed, and then visualized using the ABC technique and 0.05% 3,3'-diamino-benzidine (DAB, Sigma, USA) as a chromogen. The slides were counterstained with hemotoxylin and mounted on cover slip. Condensed and fragmented nuclei with the brown label were considered apoptotic when visualized by light microscopy. The numbers of TUNEL positive cells were counted by a pathologist: at×200 magnification, 30 fields/section. The TUNEL-positive cells were counted separately in the gray and white matter at ten axial levels.
Hoechst staining
For morphological examination of apoptotic changes, cells were stained with Hoechst 33342 (Calbiochem, San Diego, USA). Human breast cancer cells plated on glass coverslips in 6-well culture plates were grown at 37 ℃ for 24h. Troglitazone was added and incubated for an additional 48 h. The cells were fixed for 15 min at room temperature in 4% paraformaldehyde and then washed with PBS. Fixed cells were incubated for 30 min at room temperature with Hoechst 33342 (1 µg/ml) and then washed with PBS. Cells were mounted onto glass slides and examined by fluorescence microscopy. Apoptotic cells were identified by the condensation and fragmentation of their nuclei. The percentage of apoptotic cells was calculated from the ratio of apoptotic cells to total cells counted. Minimums of 500 cells were counted for each treatment.
RESULTS AND DISCUSSION
Troglitazone induces expression of PPARγ in MDA-MB-231 cells
Chemopreventive effect of PPARγ ligands on human cancers is dependent on PPARγ expression in cancer cells. Breast adenocarcinoma cells from patients expressed high levels of PPARγ protein compared to normal breast epithelial cells. PPARγ protein expression was also identified in breast cancer cell lines including MCF-7 and MDA-MB-231.(23) First, we determined whether PPARg ligand induces PPARg expression in ERα-negative breast cancer cells. To evaluate the effects of PPARg ligand on PPARg expression, MDA-MB-231 cells were cultured with various concentrations of troglitazone, a relatively selective PPARg ligand. Exposure to troglitazone for 48 h produced a dose-dependent increase of PPARg protein, as measured by Western blot (Fig 1). This result was similar to other hepatoma cancer cells responding to troglitazone.(24) These results indicate that PPARg-regulated genes play a pivotal role in the carcinogenesis of ERα-negative MDA-MB-231 breast cancer cells.
Troglitazone inhibits cell growth of MDA-MB-231 cells
In order to investigate the effect of PPARγ ligands on cell proliferation of ERα-negative MDA-MB-231 cells, the cells were seeded into 96-well culture plates at a density of 1×104 cells/well. MDA-MB-231 breast cancer cells were treated with three types of PPARγ ligands for 48 h at various concentrations including rosiglitazone, ciglitazone, and troglitazone. Cell proliferation was determined by MTT assay. PPARγ ligands inhibited the cell proliferation of MDA-MB-231 breast cancer cells in a dose-dependent manner (Fig 2). Troglitazone strongly inhibited the cell proliferation of ERα-negative MDA-MB-231 breast cancer cells. Conversely, rosiglitazone and ciglitazone had weaker effects on the MDA-MB-231 breast cancer cells compared with troglitazone.
Troglitazone induces G1 arrest of MDA-MB-231 cells
To further investigate the inhibitory effect of troglitazone on the proliferation of MDA-MB-231, cell cycle analysis was performed. MDA-MB-231 cells were cultured with various concentrations of troglitazone for 48 h. DNA content of the nuclei of MDA-MB-231 cells was analyzed by flow cytometry (Fig 3). In troglitazone-treated MDA-MB-231 cells, the percentage of G1 phase was maintained in elevated levels, indicating that troglitzone induces G1 arrest in ERα-negative MDA-MB-231 cells. Troglitazone induces G1 cell cycle arrest in ERα-positive MCF-7 cells.(19) These results suggest that troglitazone-mediated G1 arrest in breast caner cells is independent of estrogen receptor.
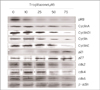
Troglitazone-mediated growth inhibition is associated with decreased levels of G1 Cdks and D-type cyclins and increased p21 and p27 protein levels in MDA-MB-231 cells
The cell cycle is tightly regulated through a complex network of positive and negative regulatory molecules including cyclin dependent kinase (Cdks), cyclin, and Cdk inhibitor (Cdki). To elucidate the role of these molecules in the inhibition of cell cycle induced by troglitazone in MDA-MB-231 breast cancer cells, protein extract was prepared from the cells treated with various concentrations of troglitazone for 48 h. Western blot was performed using antibodies against pRb, cyclin D1, cyclin D2, cyclin D3, p21, p27, cdk2, Cdk4 and Cdk6. As shown in Fig 4, troglitazone treatment dose-dependently caused a marked decrease in pRb, cyclin D1, cyclin D2, cyclin D3, cdk2, Cdk4 and Cdk6 expression and a significant increase in p21 and p27 expression.
Troglitazone induces apoptosis in MDA-MB-231 cells
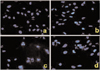
Apoptosis is a distinctive form of cell death that can result in the deletion of specific cell populations during physiologic processes. The growth inhibition by troglitazone treatment in MDA-MB-231 cells appeared to occur dependently of apoptosis. The ability of troglitazone to induce apoptosis in MDA-MB-231 breast cancer cells was initially determined by the DNA fragmentation assay based on TUNEL staining and Hoechst staining. When MDA-MB-231 breast cancer cells were exposed to various concentrations of troglitazone for 48 h, they exhibited DNA fragmentation positive cells on TUNEL staining (Fig 5).
Apoptotic cells by TUNEL staining elevated from 2.5-fold of the control level at 10 µM, to 3.1-fold at 50 µM and to 3.5-fold at 75 µM. Furthermore, MDA-MB-231 cells exposed to troglitazone exhibited typical morphological changes of apoptosis including cytoplasmic and nuclear shrinkage, chromatin condensation and fragmentation after staining with Hoechst 33342 (Fig 6).
CONCLUSION
This study addresses for the first time ERα-negative breast cancer cell growth and apoptosis may be modulated through PPARγ. MDA-MB-231 cells exposed to troglitazone showed a G1 cell cycle arrest as well as induction of morphological changes characteristic of apoptosis. Moreover, troglitazone caused significantly increase in p21 and p27 expression.
Our results suggest that troglitazone has potent effect on ERα-negative breast cancer growth and may prove to have a role in hormone therapy-resistant breast cancer treatment /prevention in the future. However, the therapeutic implications of the troglitazone growth-inhibitory effect on ERα-negative human breast cancer cells still need to be studied. Further research is necessary to establish possible therapeutic approaches using troglitazone or modified analogs of the thiazolidinedione class of drugs.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download