Abstract
Purpose
Survivin is a member of the inhibitor of apoptosis (IAP) protein family, and it is involved in the regulation of cell division. The over-expression of survivin has been reported to be associated with the parameters for a poor prognosis in most human cancers, including lung, breast, colon, stomach, esophagus, pancreas, etc. In this study, we examined the expression of a member of a novel IAP protein family, survivin, in breast cancer and its association with tumor cell apoptosis and the overall prognosis.
Methods
80 cases of formalin-fixed paraffin-embedded breast cancer tissue were immunostained with, using polyclonal survivin (Novus Biologicals, Littleton, USA), monoclonal bcl-2 (DAKO, Carpinteria, USA), and monoclonal p53 antibodies (DAKO, Carpinteria, USA). The histochemical method used for the analysis of apoptosis was based on ApopTag® Peroxidase In Situ OligoLigation (ISOL) Apoptosis Detection Kit (CHEMICON International Inc. Temecula, USA).
Results
Immunohistochemical analysis showed that cytoplasmic survivin expression was positive in 43 of 80 cases (53.8%) of breast carcinomas and it was positive for 70% of the cases that showed a bcl-2 expression tumors. Statistical analysis revealed that the survivin expression was correlated with lymph node metastasis, the tumor stage, and the histological grade. Although the survivin expression was not correlated with p53 mutations, the survivin positive cases were associated with a bcl-2 expression (p=0.015) and a reduced apoptotic index (p=0.024). On the Cox proportional hazard model analysis, the apoptotic index was not identified as a significant independent predictor of overall survival (p=0.072), although the patients with a low apoptotic index (<0.2%) had a worse survival rates than those patient in the group with a high apoptotic index (≥0.2%).
Figures and Tables
Fig 1
Immunohistochemical examination for survivin. (A) The tumor cells of infiltrating ductal carcinoma show positive reaction in survivin (×100). (B) The cytoplasm of atypical cells in moderate epithelial hyperplasia shows strong positive reaction in survivin (×100). (C) The metastatic tumor cells of infiltrating ductal carcinoma lymph node show positive reaction in survivin (×100).
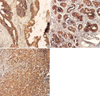
Fig 2
Histochemical staining for bcl-2 and apoptotic tumor cells. (A) bcl-2 expression in breast cancers (×40). (B) in situ oligiligation reaction (ISOL) for apoptosis (arrows: ×200).
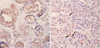
Fig 3
Kaplan-Meier survival curve according to cytoplasmic survivin expression (A) and high vs. low Apoptotic Index (B).

Table 3
Correlation between clinicopathological factors and expression of survivin, bcl-2 in breast cancer (n=80)

References
1. Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas(CD95), Bax, caspases, and anti-cancer drugs. Cancer Res. 1998. 58:5315–5320.
2. Jang JH, Kim TY, Kim SY, Baek MJ, Oh MH, Kim EH, et al. Expression of survivin in patients with breast cancer. J Korean Breast Cancer Soc. 2004. 7:236–243.

3. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term folloe-up. Histopathology. 1991. 19:403–410.

4. Kerr JF, Gobe GC, Winterford CM, Harmon BV. Anatomical methods in cell death. Methods Cell Biol. 1995. 46:1–27.
6. Palumbo A, Yeh J. Apoptosis as a basic mechanism in the ovarian cycle: follicular atresia and Iuteal regression. J Soc Gynecol Invest. 1995. 2:565–573.

7. Lipponen PK, Aaltomaa S, Kosma VM, Syrjanen K. Apoptosis in breast cancer as related to histopathological charateristics and prognosis. Eur J Cancer. 1994. 30A:2068–2073.
8. Tormanen U, Eerola AK, Rainio P, Vahakangas K, Soini Y, Sormunen R, et al. Enhanced apoptosis predicts shortened survival in non-small cell lung carcinoma. Cancer Res. 1995. 55:5595–5602.
10. Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chomosome breakpoint of neoplastic B cell with the t(14;18) chromosome translocation. Science. 1984. 226:1097–1099.

11. Yang E, Korsmeyer SJ. Molecular thanatoposis: a discourse on the bcl-2 family and cell death. Blood. 1996. 88:386–401.
12. Cory S. Regulation of lymphocyte survival by the bcl-2 gene family. Annu Rev Immunol. 1995. 13:513–543.

13. Leek RD, Kaklamanis L, Pezzella F, Gatter KC, Harris AL. bcl-2 in normal human breast and carcinoma, associated with estrogen receptorpositive, epidermal growth factor-negative tumors and in situ cancer. Br J Cancer. 1994. 69:135–139.

14. Park SH, Kim H, Song BJ. Down regulation of bcl2 expression in invasive ductal carcinomas is both estrogen and progesterone-receptor dependent and associated with poor prognostic factors. Pathol Oncol Res. 2002. 8:26–30.

15. Castiglione F, Sarotto I, Fontana V, Destefanis M, Venturino A, Ferro S, et al. Bcl2, p53 and clinical outcome in a series of 138 operable breast cancer patients. Anticancer Res. 1999. 19:4555–4563.
16. Ritter JH, Dresler GM, Wick MR. Expression of bcl-2 protein in stage TINOMO non-small cell lung carcinoma. Hum Pathol. 1995. 26:1227–1232.
17. Inada T, Kikuyama S, Ichikawa A, Igarashi S, Ogata Y. Bcl-2 expression as a prognostic factor of survival of gastric carcinoma. Anticancer Res. 1998. 18:2003–2010.
18. Hellemans P, Dam PA, Weyler J, Oosteroma AT, Buytaert P, Marck EV. Prognostic value of bcl-2 expression in invasive breast cancer. Br J Cancer. 1995. 72:354–360.

19. Pezzella F, Turley H, Kuzu I, Tungekar MF, Dunnill Ms, Pierce CB, et al. Bcl-2 proteinin non-small cell lung caricnoma. N Eng J Med. 1993. 329:690–694.
20. Crook NE, Clem RJ, Miller LK. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J Virol. 1993. 67:2168–2174.

21. Birnbaum MJ, Clem RJ, Miller LK. An apoptosis-inhibiting gene from a nuclear polyhedrosis virus encoding a polypeptide with Cys/His sequence motifs. J Vitol. 1994. 69:2521–2528.

22. Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death protease. Nature. 1997. 388:300–304.

23. Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nature Med. 1997. 3:917–921.

24. Verdecia MA, Huang H, Dutil E, Kaiser DA, Hunter T, Noel JP. Structure of the human anti-apoptotic protein survivin reveals a dimeric arrangement. Nat Struct Biol. 2002. 7:620–623.
25. Hague A, Moorghen M, Hicks D, Champman M, Paraskeva C. Bcl-2 expression in human colorectal adenomas and carcinomas. Oncogene. 1994. 9:3367–3370.
26. LaCasse EC, Baird S, Korneluk RG, Mackenzie AE. The inhibitor of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998. 17:3247–3259.

27. Deveraux QL, Reed JC. IAP family proteing-suppressors of apoptosis. Gene Day. 1999. 12:239–252.
28. Olie RA, Simoes-Wust AP, Baumann B, Leech SH, Fabbro D, Stahel RA, et al. A novel antisense oligonucleotide targeting survivin expression induces apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer Res. 2000. 60:2805–2809.
29. Monzo M, Rosell R, Felip E, A studillo J, Sanchez JJ, Maestre J, et al. A novel anti-apoptosisgene:Re-expression of survivin messenger RNA as a prognosis marker in non-small cell lung cancers. J Clin Oncol. 1999. 17:2100–2104.
30. Adida C, Berrebi D, Peuchmaur M, Reyes-Mugica M, Altieri DC. Anti-apoptosis gene, survivin, and prognosis of neuroblastoma. Lancet. 1998. 351:882–883.

31. Okada E, Murai Y, Matsui K, Isizawa S, Cheng C, Masuda M, et al. Survivin expression in tumor cell nuclei is predictive of a favorable prognosis in gastric cancer patients. Cancer Lett. 2001. 163:109–116.

32. Ito T, Shiraki K, Sugimoto K, Yamanaka T, Fujikawa K, Ito M, et al. Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology. 2000. 31:1080–1085.

33. Kennedy SM, O'Driscoll L, Purcell R, Fitz-Simons N, McDermott EW, Hill AD, et al. Prognostic importance of survivin in breast cancer. Br J Cancer. 2003. 88:1077–1083.

34. Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998. 58:5071–5074.
35. Lu CD, Altieri DC, Tanigawa N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res. 1998. 58:1808–1812.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download