Abstract
The role of the treatment for latent tuberculosis infection (LTBI) has been underscored in the intermediate tuberculosis (TB) burden countries like South Korea. LTBI treatment is recommended only for patients at risk for progression to active TB―those with frequent exposure to active TB cases, and those with clinical risk factors (e.g., immunocompromised patients). Recently revised National Institute for Health and Care Excellence (NICE) guideline recommended that close contacts of individuals with active pulmonary or laryngeal TB, aged between 18 and 65 years, should undergo LTBI treatment. Various regimens for LTBI treatment were recommended in NICE, World Health Organization (WHO), and Centers for Disease Control and Prevention guidelines, and superiority of one recommended regimen over another was not yet established. Traditional 6 to 9 months of isoniazid (6H or 9H) regimen has an advantage of the most abundant evidence for clinical efficacy―60%–90% of estimated protective effect. However, 6H or 9H regimen is related with hepatotoxicity and low compliance. Four months of rifampin regimen is characterized by less hepatotoxicity and better compliance than 9H, but has few evidence of clinical efficacy. Three months of isoniazid plus rifampin was proved equivalence with 6H or 9H regimen in terms of efficacy and safety, which was recommended in NICE and WHO guidelines. The clinical efficacy of isoniazid plus rifapentine once-weekly regimen for 3 months was demonstrated recently, which is not yet introduced into South Korea.
Mycobacterium tuberculosis is carried in airborne droplet nuclei coughed into the atmosphere by patients with active tuberculosis (TB). The infected droplet nuclei remain suspended in the air and can be transmitted when inhaled1. About 90% of infected patients can normally respond to M. tuberculosis infection by granuloma formation, without manifesting clinical symptoms of active TB disease, in a condition known as latent tuberculosis infection (LTBI). However, in 10% of infected patients with various immunological conditions, dormant mycobacterium begins to grow and LTBI develops into active TB2. Transmission of TB to the community occurs in the endless repetitive cycle of contact, infection, and development of active disease. World Health Organization (WHO) guidelines define LTBI as state of persistent immune response to stimulation by M. tuberculosis antigens without evidence of clinically manifested active TB3. Current diagnostic tests for M. tuberculosis infection cannot distinguish between completely cured TB infections, active and latent TB. Therefore, in addition to positive testing for M. tuberculosis infection, proper diagnosis of LTBI requires that the presence of active TB disease must be excluded.
To eliminate TB worldwide by 2050, which means annual incidence of TB less than one case per million people, the incidence of TB must decrease at 20% per year on average from 2015 to 20504. That target can only be achieved with effective treatment of active TB combined with treatment of LTBI that can progress to active TB. In most high TB burden countries, where the majority of cases result from recent infection, earlier detection, accurate diagnosis and achieving high cure rates is the pillar of their anti-tuberculosis strategy. Where the prevalence of human immunodeficiency virus (HIV) infection is high, as in South Africa, preventive therapy with isoniazid or other anti-tuberculosis drug among people infected with HIV is vital in addition to case finding and case management strategy. Where the prevalence of HIV infection is low (<1%), as in China, mass preventive therapy for LTBI plays an important role in TB elimination. In low TB burden countries, maintaining low transmission rate, preventing TB cases which result from remote infection among native born population, especially for the elderly is important. Because the majority of TB cases occur among the immigrants, screening of immigrants is another important anti-tuberculosis strategy in low TB burden countries. Under these circumstances, WHO recommended treatment of patients with LTBI at high risk of progressing to active TB, in high- and middle-income countries with annual TB incidence below 100 cases per 100,000 persons3.
South Korea has the disproportionately high TB burden among the member countries of the Organization for Economic Co-operation and Development (OECD)5. One of the reasons for the relatively high burden of TB in South Korea is high prevalence of LTBI in the elderly population6. The number and proportion of notified TB patients over 65 years of age is increasing despite the decrease in total incidence of TB, rising from 6,547 new TB cases out of 34,123 total new TB cases (19.2%) in 2001 to 12,328 new TB cases out of 30,892 total new TB cases (39.9%) in 20167. Increasing TB burden in elderly is related with aging society8. LTBI prevalence among the elderly population in South Korea is estimated to be high. In one study, among the participants >60 years of age without radiographic evidence of prior TB, 67.2% were diagnosed as LTBI, based on interferon-γ release assay result9. TB in elderly population in low burden countries is associated with endogenous reactivation rather than recent transmission10. This phenomenon is also observed in many other intermediate burden countries, where the risk of transmission tends to decrease11.
In South Korea, TB incidence decreased from 100.8 cases per 100,000 people in 2011 to 76.8 cases per 100,000 people in 20167. Until recently, LTBI treatment was not a possible option in South Korea for controlling active TB. However, the 2011 Korean guidelines on TB recommended treatment of LTBI in patients with close contacts of active TB below the age of 35 years, and non-contacts with risk factor of development to active TB. The revised 2014 guidelines recommended treatment of LTBI in more specific clinical situations, especially in immunocompromised patients.
After confirming isoniazid's clinical efficacy in TB treatment in the 1950s, isoniazid has been essential for TB therapy. In 1954, Dr. Lincoln at Bellevue Hospital in New York City found that children with primary tuberculosis who had undergone isoniazid treatment had no complication like meningitis, and suggested that children with recent conversion of tuberculin skin test (TST), so called asymptomatic primary tuberculosis should be treated with isoniazid for a year―a period in which meningitis most likely to develop12. In a multi-clinic placebo controlled clinical trial organized by U.S. Public Health Service in which 2,750 asymptomatic children with recent TST conversion were enrolled, 1-year isoniazid preventive therapy made 94% reduction in the incidence of TB during a year of treatment, and 70% reduction over following 8-year period13. In 1965, American Thoracic Society adopted preventive chemotherapy for LTBI14.
Considering that only 10% of LTBI proceeds to active TB, LTBI treatment is recommended only for LTBI patients at risk of progressing to active TB. In other words, diagnostic test for LTBI is recommended only for those who need treatment when LTBI is confirmed15. Patients at risk for progression to active TB can be classified into two groups: those with frequent exposure to active TB cases and those with clinical risk of developing active TB. The former includes persons in close contact to active cases, for example, family contacts or employees in correctional and nursing facilities. A typical example of the latter is people with HIV. Recommended indications for diagnosis and treatment of LTBI according to the current guidelines of National Institute for Health and Care Excellence (NICE, UK), WHO, and Centers for Disease Control and Prevention (CDC, USA) are listed in Table 1.
In United Kingdom, TB cases amongst immigrants comprise over two-thirds of total TB cases16. Reactivation of TB at least 6 years after immigration to United Kingdom is increasing, and accounts for 60% of total TB cases amongst immigrants. It is recommended that immigrants from high TB burden countries (prevalence higher than 150 patients per 100,000 people) below the age of 65 years should be tested and treated for LTBI, according to the revised NICE guidelines17.
Also in United States, active TB among foreign-born persons accounted for two-thirds of total TB cases in 201518. CDC guideline classified persons who have immigrated from TB-endemic regions of the world as persons at risk for developing TB disease, and recommended test and treatment for LTBI19.
In contrast to NICE guidelines, WHO guidelines recommended against patients with diabetes mellitus and alcoholics undergoing test and treatment for LTBI. Whereas CDC guidelines recommended test and treatment for LTBI of those who are underweight, WHO guidelines opposed that.
Guidelines published by NICE, WHO, and CDC suggested treatment for LTBI in close contacts of persons with TB disease (Table 1). Close contacts of persons with TB disease in all age groups were required to undergo diagnostic tests for active TB; however, additional diagnostic testing and treatment for LTBI were recommended only for those below the age of 35 years, according to the previous version of NICE guideline (2006). However, according to CDC guideline, diagnosis and treatment of LTBI was not limited to specific age groups; additionally, targeted and personalized treatment was recommended, after estimating the patients' lifelong benefit and risk of LTBI treatment19. Recently, revised NICE guideline (2016) recommended that close contacts of active pulmonary or laryngeal TB, 18–65 years old, should undergo diagnosis and treatment of LTBI, after excluding active TB17. Revised NICE guideline, taking into account an economic model analysis on the risk and benefit of LTBI treatment, amended the age limit of LTBI treatment indication from 35 years old to 65 years old and recommended LTBI treatment for persons over 35 years old only when there is low risk for hepatotoxicity.
Chest radiography should be performed before LTBI treatment to exclude active TB disease3. LTBI patients, recently exposed to active cases, were advised to refer to the drug susceptibility test results of the index case. Baseline blood test should be performed before treatment, and regular blood chemistry follow-up is needed in case of hepatotoxicity risk. NICE guidelines recommended baseline blood test for hepatitis B and C. If active TB is diagnosed during LTBI treatment, the LTBI regimen should be amended to standard treatment regimen for active TB, including the drug used in LTBI treatment17. Recommended regimens according to the current guidelines of NICE (UK), WHO, and CDC (USA) are listed in Table 2.
Isoniazid treatment is advantageous due to the considerable clinical experience and low cost. In several placebo-controlled trials, 12 months isoniazid monotherapy (12H)'s average protective effect for TB was approximately 60% during the observation period2021. When analysis was limited to participants with good compliance, the efficacy increased to 93%. In one report, the protective effect of 12H regimen lasted 19 years22. Optimal duration of taking isoniazid remains controversial. In International Union Against Tuberculosis (IUAT) trial published in 1982, 27,830 participants with fibrotic pulmonary lesions but not active TB were randomized to each group taking isoniazid for 3 months, 6 months, 12 months, and placebo21. After 5 years of follow-up, the risk for developing active TB compared with placebo was reduced by 21%, 65%, and 75%, respectively. Additional cost-effect analysis of IUAT trial had concluded that 6 months of isoniazid (6H) regimen is more cost effective than 3 months of isoniazid regimen or 12H regimen23. According to these results, American Thoracic Society guideline published in 1994 recommended that 6H regimen is nearly as good as 12H regimen24.
The evidence of 9 months of isoniazid (9H) regimen was derived from retrospective re-analysis of Bethel Isoniazid Studies22. In this study, participants were randomized to either placebo or 12H group, and after a year, all participants in both groups were supplemented with isoniazid. According to the patients' various compliance, total duration of actually taking isoniazid ranged from 0 to 24 months. In a plot of TB cases per 100 persons against the total duration of taking isoniazid, steep slope of the decline curve became flattened after 9–10 months suggesting that there is little additional protective effect after 9–10 months of taking isoniazid25. Both WHO and CDC guidelines recommended 6H or 9H regimens for LTBI treatment, in the absence of a direct comparison between 6H and 9H regimens319. 9H regimen is superior to 6H regimen, in terms of clinical efficacy, but 6H regimen is more cost-effective with better compliance. Based on the equivalence between 6H and 9H, revised NICE guideline recommended 6H regimen, because it is shorter17. NICE guideline also recommended pyridoxine supplementation with all isoniazid regimens. 6H regimen was also recommended in case of potential adverse effects due to drug interactions with rifamycins, for example, in HIV patients taking antiretroviral treatment.
A major adverse effect of isoniazid is hepatotoxicity that increases with older age―the estimated incidence of hepatotoxicity is 0.1%–0.2% in those who under 20 years of age, 0.3% in 20–34 years of age, 0.5% in 35–49 years of age, 1%–3% in 50–64 years of age, and 2%–5% in over 65 years of age26. Other risk factors for hepatotoxicity include preexisting liver disease, concomitant use of other hepatotoxic medications, prior isoniazid-related hepatotoxicity, and regular alcohol consumption27.
As the isoniazid regimen had shortcomings of suboptimal compliance and hepatotoxicity, rifampin was proposed as an alternative agent for treatment of LTBI which can shorten the treatment duration28. In one experimental study with LTBI mouse model, the proportions of mice with positive spleen culture which was done at 6 months after treatment with each regimen (6H, 3 months of rifampin [3R], 2 months of rifampin plus pyrazinamide [2RZ], or 2 months of isoniazid plus rifampin plus pyrazinamide) were 100%, 60%, 56%, and 95%, respectively, suggesting that 3R or 2RZ regimen were more effective than 6H regimen29. There is no large scaled randomized controlled trial (RCT) on comparing the long term protective effect of rifampin with that of isoniazid among the general LTBI patients, not in a specific disease group. The only RCT which can be a clue for estimation of efficacy is that performed among silicosis patients in Hong Kong. In this study, the protective effects of 3R, 3 months of isoniazid plus rifampin (3HR), and 6H regimens were 63%, 48%, and 41%, respectively30. When it comes to compliance and hepatotoxicity, 4 months of rifampin (4R) regimen showed better compliance with less hepatotoxicity, and was more cost-effective when compared with 9H regimen in a meta-analysis that included two RCTs and two retrospective studies31. 4R regimen is preferred in United States when the index case is isoniazid-resistant19. Currently, large scaled RCT is under way that compares long term protective efficacy of 4R with 9H regimen (https://clinicaltrials.gov/ct2/show/NCT00931736).
The regimen of isoniazid plus rifampin was introduced to United Kingdom in 1981 for the prophylaxis of children with exposure history and immigrants with LTBI, especially from India. The reason for adding rifampin was high (6%) incidence of isoniazid resistance in index cases from India. The original treatment duration was 9 months (9 months of isoniazid plus rifampin) in 1981, and was gradually shortened to 3 months (3HR) in 1989. Though there was no comparison with isoniazid monotherapy, regimen of isoniazid plus rifampicin was successful in reducing TB burden of community, with only a few cases of adverse effect32. With this evidence, British Thoracic Society recommended a 3-month regimen of rifampin plus isoniazid as an alternative short-course therapy for LTBI in 199833. In a meta-analysis study of five RCTs comparing 3HR regimen with isoniazid monotherapy for 6 to 12 months, both regimens were equivalent in terms of efficacy and safety34. In a network meta-analysis study of RCTs comparing each regimen, 3HR was an effective regimen along with 3R or 4R regimen35. Consistent with the results of this study, NICE guidelines recommended 3HR regimen as a standard regimen17, and WHO guidelines published in 2015 also recommended 3HR regimen3. In a retrospective study conducted in South Korea which compared 9H, 4R, and 3HR regimens among the patients receiving anti-tumour necrosis factor therapy, completion rates were 73.8%, 87.1%, and 94.2%, respectively, with no difference in adverse drug reaction36.
Rifapentine is a rifamycin derivative with a long half-life and greater potency against M. tuberculosis than rifampin. Since pharmacokinetic study of rifapentine with murine LTBI model had proved efficacy of regimen of once weekly, once weekly isoniazid plus rifapentine regimen was tested in several studies with murine LTBI model, and showed superior or at least equivalent efficacy when compared with isoniazid monotherapy3738. The most important RCT which compared directly observed once-weekly isoniazid plus rifapentine for 3 months (3HP) with conventional daily self-administered 9H regimen was PREVENT TB trial conducted by the Tuberculosis Trials Consortium in Brazil, Canada, Spain, and the United States39. In this study, 3HP regimen was noninferior to 9H in preventing active TB in 33 months of follow-up period. Treatment completion rates were higher with 3HP regimen compared to 9H regimen (82% vs. 69%, respectively), and the rate of treatment discontinuation due to hepatotoxicity was low (0.3% vs. 2.0%). But the rate of permanently stopping treatment due to serious adverse effect was higher with 3HP regimen (4.9% vs. 3.7%). Hypersensitivity reaction was common serious adverse effect that cuased permanent stop of the treatment, which was higher with 3HP regimen (2.9% vs. 0.4%). The rate of hepatotoxicity was lower with 3HP regimen (0.4% vs. 1.9%). After the end of PREVENT TB trial, significant systemic drug reactions (SDR) that occurred during the treatment period were reviewed40. Of the 138 patients in 3HP arm who reported SDRs, 87 (63%) had flu-like syndrome and 23 (17%) had cutaneous reactions. Symptoms occurred after a median of three doses, and 4 hours after administration of the dose. Median time to resolution was 24 hours.
Treatment of LTBI is important for the elimination of TB, especially in intermediate burden countries like South Korea. Although the incidence of TB is decreasing after launching National Tuberculosis Control Programs, South Korea is still the highest burden TB country among the OECD countries. South Korea has unique epidemiologic features of TB due to rapid economic growth, rapid population aging, and rapid urbanization. The burden of TB among the elderly population is increasing, which indicates the preventive strategies for reactivation from remote TB infection is needed. To reduce TB burden, more accurate active diagnosis and better treatment of LTBI are needed in addition to increasing early detection and treatment completion rate for active TB.
After the efficacy of isoniazid had been proved in treatment of LTBI, various regimens were tested for their efficacy and safety. RCTs comparing each regimen are in progress. Guidelines published by NICE, WHO, and CDC were based on their own evidences. Before government's extension of LTBI treatment policy, various prospective and retrospective studies are needed to tailor the national strategy for TB elimination in South Korea. Those studies will serve as the basis for the development of National Tuberculosis Control Programs.
References
1. Ahmad S. Pathogenesis, immunology, and diagnosis of latent Mycobacterium tuberculosis infection. Clin Dev Immunol. 2011; 2011:814943. PMID: 21234341.
2. Ahmad S. New approaches in the diagnosis and treatment of latent tuberculosis infection. Respir Res. 2010; 11:169. PMID: 21126375.

3. World Health Organization. Guidelines on the management of latent tuberculosis infection [Internet]. Geneva: World Health Organization;2015. cited 2017 Jun 1. Available from: http://www.who.int/tb/publications/latent-tuberculosis-infection/en/.
4. Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health. 2013; 34:271–286. PMID: 23244049.

5. World Health Organization. Global tuberculosis report 2016 [Internet]. Geneva: World Health Organization;2016. cited 2017 Jun 1. Available from: http://www.who.int/tb/publications/global_report/en/.
6. Kim JH, Yim JJ. Achievements in and challenges of tuberculosis control in South Korea. Emerg Infect Dis. 2015; 21:1913–1920. PMID: 26485188.

7. Korea Centers for Disease Control and Prevention. Annual report on the notified tuberculosis patients in Korea, 2016. Cheongju: Korea Centers for Disease Control and Prevention;2017.
8. Park YK, Park YS, Na KI, Cho EH, Shin SS, Kim HJ. Increased tuberculosis burden due to demographic transition in Korea from 2001 to 2010. Tuberc Respir Dis. 2013; 74:104–110.

9. Jeong YJ, Yoon S, Koo HK, Lim HJ, Lee JS, Lee SM, et al. Positive tuberculin skin test or interferon-gamma release assay in patients with radiographic lesion suggesting old healed tuberculosis. J Korean Med Sci. 2012; 27:761–766. PMID: 22787371.

10. Small PM, Hopewell PC, Singh SP, Paz A, Parsonnet J, Ruston DC, et al. The epidemiology of tuberculosis in San Francisco: a population-based study using conventional and molecular methods. N Engl J Med. 1994; 330:1703–1709. PMID: 7910661.
11. Mori T, Leung CC. Tuberculosis in the global aging population. Infect Dis Clin North Am. 2010; 24:751–768. PMID: 20674802.

12. Lincoln EM. The effect of antimicrobial therapy on the prognosis of primary tuberculosis in children. Am Rev Tuberc. 1954; 69:682–689. PMID: 13148531.
13. Jenkins D, Davidson FF. Isoniazid chemoprophylaxis of tuberculosis. Calif Med. 1972; 116:1–5.
14. Runyon EH. Preventive treatment in tuberculosis: a statement by the Committee on Therapy, American Thoracic Society. Am Rev Respir Dis. 1965; 91:297–298. PMID: 14253184.
15. Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000; 161(4 Pt 2):S221–S247. PMID: 10764341.
16. Public Health England. Tuberculosis in England, 2016 report [Internet]. London: Public Health England;2016. cited 2017 Jun 1. Available from: https://www.gov.uk/government/publications/tuberculosis-in-england-annual-report.
17. National Institute for Health and Clinical Excellence (NICE). Tuberculosis (NICE guideline 33) [Internet]. London: NICE;2016. cited 2017 Jun 1. Available from: https://www.nice.org.uk/guidance/ng33.
18. Salinas JL, Mindra G, Haddad MB, Pratt R, Price SF, Langer AJ. Leveling of tuberculosis incidence: United States, 2013-2015. MMWR Morb Mortal Wkly Rep. 2016; 65:273–278. PMID: 27010173.
19. Centers for Disease Control and Prevention. Latent tuberculosis infection: a guide for primary health care providers [Internet]. Atlanta: Centers for Disease Control and Prevention;2013. cited 2017 Jun 1. Available from: https://www.cdc.gov/tb/publications/ltbi/default.htm.
20. Falk A, Fuchs GF. Prophylaxis with isoniazid in inactive tuberculosis: a Veterans Administration Cooperative Study XII. Chest. 1978; 73:44–48. PMID: 340155.
21. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. International Union Against Tuberculosis Committee on Prophylaxiss. Bull World Health Organ. 1982; 60:555–564. PMID: 6754120.
22. Comstock GW, Baum C, Snider DE Jr. Isoniazid prophylaxis among Alaskan Eskimos: a final report of the bethel isoniazid studies. Am Rev Respir Dis. 1979; 119:827–830. PMID: 453704.
23. Snider DE Jr, Caras GJ, Koplan JP. Preventive therapy with isoniazid: cost-effectiveness of different durations of therapy. JAMA. 1986; 255:1579–1583. PMID: 3081740.

24. Bass JB Jr, Farer LS, Hopewell PC, O'Brien R, Jacobs RF, Ruben F, et al. Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and The Centers for Disease Control and Prevention. Am J Respir Crit Care Med. 1994; 149:1359–1374. PMID: 8173779.

25. Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis. 1999; 3:847–850. PMID: 10524579.
26. Public Health Agency of Canada. Canadian tuberculosis standards, 7th edition [Internet]. Ottawa: Public Health Agency of Canada;2014. cited 2017 Jun 1. Available from: http://www.phac-aspc.gc.ca/tbpc-latb/pubs/tb-canada-7/index-eng.php.
27. Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006; 174:935–952. PMID: 17021358.

28. Farer LS. Chemoprophylaxis. Am Rev Respir Dis. 1982; 125(3 Pt 2):102–107. PMID: 7041718.
29. Lecoeur HF, Truffot-Pernot C, Grosset JH. Experimental short-course preventive therapy of tuberculosis with rifampin and pyrazinamide. Am Rev Respir Dis. 1989; 140:1189–1193. PMID: 2817579.

30. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. Am Rev Respir Dis. 1992; 145:36–41. PMID: 1731596.
31. Ziakas PD, Mylonakis E. 4 months of rifampin compared with 9 months of isoniazid for the management of latent tuberculosis infection: a meta-analysis and cost-effectiveness study that focuses on compliance and liver toxicity. Clin Infect Dis. 2009; 49:1883–1889. PMID: 19911936.

32. Ormerod LP. Rifampicin and isoniazid prophylactic chemotherapy for tuberculosis. Arch Dis Child. 1998; 78:169–171. PMID: 9579163.

33. Chemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998. Joint Tuberculosis Committee of the British Thoracic Society. Thorax. 1998; 53:536–548. PMID: 9797751.
34. Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis. 2005; 40:670–676. PMID: 15714411.

35. Stagg HR, Zenner D, Harris RJ, Munoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med. 2014; 161:419–428. PMID: 25111745.
36. Park SJ, Jo KW, Yoo B, Lee CK, Kim YG, Yang SK, et al. Comparison of LTBI treatment regimens for patients receiving anti-tumour necrosis factor therapy. Int J Tuberc Lung Dis. 2015; 19:342–348. PMID: 25686145.

37. Zhang T, Zhang M, Rosenthal IM, Grosset JH, Nuermberger EL. Short-course therapy with daily rifapentine in a murine model of latent tuberculosis infection. Am J Respir Crit Care Med. 2009; 180:1151–1157. PMID: 19729664.

38. Miyazaki E, Chaisson RE, Bishai WR. Analysis of rifapentine for preventive therapy in the Cornell mouse model of latent tuberculosis. Antimicrob Agents Chemother. 1999; 43:2126–2130. PMID: 10471552.

39. Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011; 365:2155–2166. PMID: 22150035.

40. Sterling TR, Moro RN, Borisov AS, Phillips E, Shepherd G, Adkinson NF, et al. Flu-like and other systemic drug reactions among persons receiving weekly rifapentine plus isoniazid or daily isoniazid for treatment of latent tuberculosis infection in the PREVENT Tuberculosis Study. Clin Infect Dis. 2015; 61:527–535. PMID: 25904367.

Table 1
Recommended indications for diagnosis and treatment of LTBI according to the current guidelines of NICE, WHO, CDC
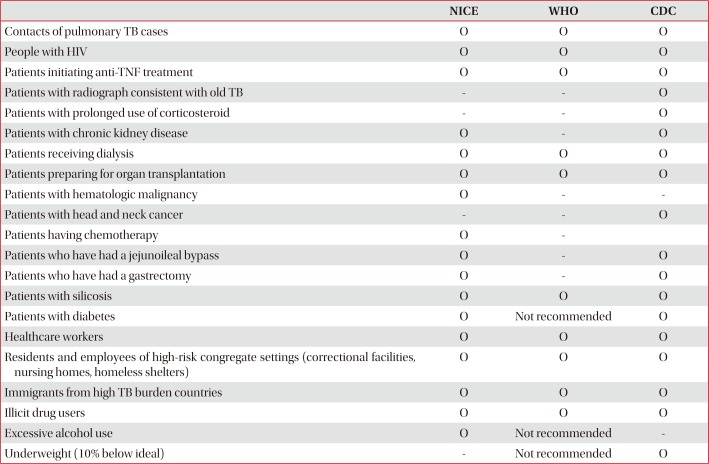
Table 2
Recommended regimens for treatment of LTBI according to the current guidelines of NICE17, WHO3, and CDC19
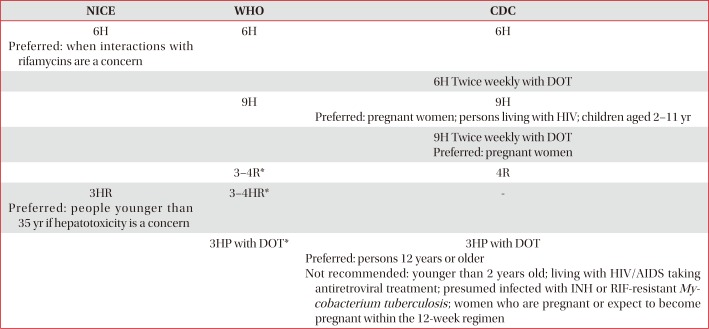
*Should be prescribed with caution to people with HIV who are on antiretroviral treatment.
LTBI: latent tuberculosis infection; NICE: National Institute for Health and Care Excellence; WHO: World Health Organization; CDC: Centers for Disease Control and Prevention; 6H: 6 months of isoniazid; DOT: directly observed treatment; 9H: 9 months of isoniazid; HIV: human immunodeficiency; 3–4R: 3–4 months of rifampin; 3HR: 3 months of isoniazid plus rifampin; 3HP: isoniazid plus rifapentine once-weekly regimen for 3 months; AIDS: acquired immunodeficiency syndrome; INH: isoniazid; RIF: rifampin.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download