Abstract
Desmoid tumors are rare soft tissue tumors considered to have locally infiltrative features without distant metastasis until now. Although they are most commonly intraabdominal, very few cases have extra-abdominal locations. The origin of intrathoracic desmoid tumors is predominantly the chest wall with occasional involvement of pleura. True intrathoracic primary desmoid tumors with no involvement of the chest wall or pleura are extremely rare. We recently experienced a case of true intrathoracic desmoid tumor presenting as multiple lung nodules at 13 years after resection of a previous intraabdominal desmoid tumor.
Desmoid tumors are rare soft tissue tumors accounting for 0.03% of all neoplasms. Histologic findings of them are benign fibrous neoplasms. They invade locally and after resection, recurrence rate is 19%-77%. Distant metastasis from them or recurrence in another distant location has not yet been confirmed12.
Desmoid tumors mainly occur in the abdomen, and extra-abdominal desmoid tumors are not frequent. The most common extra-abdominal locations are the chest wall, shoulder girdle, thigh, and head and neck1234. Most intrathoracic desmoid tumors arise from the chest wall and extend intrathoracically. Occasionally, they originate from pleura. Only one case of true intrathoracic desmoid tumors not involving the chest wall or pleura has been reported567. Intrathoracic desmoid tumors are also not known to form lung nodules. It is extremely rare that a desmoid tumor occurred in an unusual form not in a location adjacent to the original site but in a distant location.
Through a search of electronic data bases, including Pubmed, there were only 27 previously reported cases of intrathoracic desmoid tumors. No case as a pulmonary nodule without chest wall or pleura involvement has been reported until now. There is only one case of intrabronchial desmoid tumor, and recurrence in distant locations other than primary sites has not been confirmed16.
We report on a case of true intrathoracic desmoid tumor presenting as multiple lung nodules and occurring 13 years after resection of a previous abdominal wall mass.
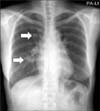
A 54-year-old woman visited our clinic for recurrent upper respiratory infection. Three years ago, she had a dry cough for weeks, and a chest radiograph showed tiny lung nodules. Although a follow-up chest radiograph was recommended, she did not return. Her new chest radiograph showed multiple lung nodules occurring at the same sites, but larger in size (Figure 1). She was hospitalized to obtain pathologic samples from the nodules for differentiation from malignancy.
She had never smoked and had no trauma or chest operation history. She entered menopause 2 years ago. Sixteen years ago, she had an operation to remove an abdominal wall mass. At that time, the mass was histologically confirmed as a desmoid tumor and no additional treatment was performed.
During our patient's recent hospitalization, she was alert and the following findings were noted in a physical examination: blood pressure, 130/80 mm Hg; pulse rate, 72 beats per minute; respiratory rate, 20 breaths per minute; and body temperature, 36.8℃. She had no prominent abnormalities on chest, abdominal, or extremity examinations. Clear breath sounds were auscultated on the thorax, and other results of the physical examination were non-specific.
A chest radiograph showed multiple lung nodules. In the chest computed tomography (CT) images, small multiple nodules were scattered throughout the whole lung field including apex and posterior segment of right upper lobe, right middle lobe, superior segment of right lower lobe (RLL), and left major fissure. Pulmonary nodules in both lungs were equal or less than 2.1 cm in diameter and a well-circumscribed round shape, with boundaries showing delayed enhancement (Figure 2). Compared to the chest radiograph that was performed 3 years ago, the pulmonary nodules grew bigger. Nodules displayed in the current chest CT seemed to be benign, not malignant. Multiple ill-defined ground glass opaque nodules were also present in both lungs (Figure 2). Lymph node enlargement in the mediastinum was not seen.
Laboratory findings were as follows: white blood cell count, 4,600/mm3; hemoglobin, 12.4 g/dL; platelet count, 230,000/mm3; aspartate aminotransferase, 27 IU/L; alanine aminotransferase, 28 IU/L; total protein, 7.0 g/dL; blood urea nitrogen, 13 mg/dL; creatinine, 0.7 mg/dL; and C-reactive protein, 0.2 mg/dL.
Fine needle aspiration biopsy (FNAB) targeting the largest nodule in the RLL revealed fibrous tissue. Microscopically, the tumor was composed of bland-looking spindle cells within abundant collagenous tissue with myxoid change (Figure 3A). Immunohistochemically, the spindle cells were positive for vimentin (Figure 3B) and negative for CD34 (Figure 3C) and smooth muscle actin (SMA). This histologic and immunohistochemical findings were compatible with desmoid tumor. In the review of the previous abdominal mass lesion, the histologic and immunohistochemical features of the lung biopsy was same to those of the abdominal mass conducted 16 years ago (Figure 3D). But medical records at that time did not exist and she knew that benign tumor was treated successfully with surgical removal alone. Moreover only a portion of the abdominal wall mass remained and the amount of lung nodule obtained through FNAB was inadequate, it was infeasible to compare both tissues by genetic testing.
Extra-abdominal desmoid tumors are known to be locally invasive but not malignant. If their progression is fast or symptoms are severe, definitive therapy should be considered. Our case, however, had very mild symptoms and her tumor was assumed to be not invasive, even though the nodules were scattered throughout the both lung. We decided to monitor her condition with no additional treatment. Three months later, a follow-up chest radiograph showed no significant interval change. In the chest CT taken at the same time, multiple lung nodules showed no interval change and multiple ill-defined ground glass opacity nodules were decreased in size. During the 12-month follow-up, she had no symptoms, recurrence, or metastasis.
In our case, the intra-abdominal desmoid tumor was resected 16 years ago. No recurrence at the operated site had been reported, but an intrathoracic desmoid tumor presenting as multiple lung nodules was casually discovered 3 years ago. Desmoid tumors very rarely sequentially occurred in different locations1.
Incidence of desmoid tumors is very low and they occur frequently in the intra-abdominal and infrequently in the extra-abdominal region. Like extra-abdominal desmoid tumors, intrathoracic desmoid tumors demonstrate variable disease courses. Although there is a possibility of malignant transformation in extra-abdominal desmoid tumors, malignant formation in intrathoracic desmoid tumors has not yet been reported48.
The exact pathogenic mechanism of desmoid tumors is not known clearly, but history of trauma including surgical scarring, genetic factors, and hormonal factors are likely causes. Approximately 25% of patients with desmoid tumors had a previous history of trauma9. Vigorous inflammatory reaction after trauma have been thought to be possible etiologies410. Desmoid tumors have been associated with some genetic diseases such as familial adenomatous polyposis or Gardner's syndrome. It is thought that genetic defects may affect generation of desmoid tumors11. In recent studies, it was confirmed that incidence of adenomatous polyposis coli and β-catenin gene mutations was high in desmoid tumors12. Another factor affecting development of desmoid tumors is hormones. Desmoid tumors mainly occur in females of reproductive age. Estrogen and progesterone receptors are often found in desmoid tumors, and regression of desmoid tumors with tamoxifen therapy have been reported113.
For the definite diagnosis of desmoid tumors, histopathological examination should be performed. In case of desmoid tumor, spindle cells are negative for CD34 but positive for vimentin, and variablly for SMA. On the other hand, solitary fibrous tumors, which need to be ruled out because they commonly arise from the pleura, are characteristically positive for CD34 and vimentin67. Inflammatory myofibroblastic tumor and IgG4-related sclerosing disease also need to be excluded because they occasionally appear as lung nodules. In our case, we could rule out them based on spindle cell proliferation without infiltrating inflammatory cells.
Currently, there is no standard treatment for desmoid tumors. Traditional treatments have included surgical resection, radiotherapy, hormone therapy, and medications. Wide surgical resection with free margin was the first-line treatment until recently. Despite complete excision, recurrence rate of desmoid tumor has been reported from 20% up to 60%14. Due to its rarity, recurrence of intrathoracic desmoid tumors is not accurately known15. The mechanisms of radiation therapy and the effects of antiestrogen and cytotoxic agents are uncertain, but in inoperable cases or those with recurrent lesions, they have usually been used14. It has also been reported that nonsteroidal anti-inflammatory drugs in conjunction with ascorbic acid or tamoxifen reduce the growth of desmoid tumors13. However, clinical merits of these treatments with adequate controlled trial data have not been confirmed. Recently, a "wait-and-see" policy has been followed as front-line management for extra-abdominal desmoid tumors, with a cumulative failure probability of 10%1415.
In our case, we diagnosed intrathoracic desmoid tumors derived from lung parenchyma and presenting as multiple lung nodules. They were not derived from the chest wall or pleura like other intrathoracic desmoid tumors and they did not resemble mass-like lesions. Contrast to their locally aggressive nature, distant metastasis of desmoid tumors has not been reported. In our case, an intra-abdominal desmoid tumor was diagnosed 16 years ago and resected, with no recurrence of abdominal mass up until her most recent visit to our clinic. However, intrathoracic desmoid tumors were casually detected at a health screening 3 years ago. Determining whether multiple lung nodules were multiple desmoid tumors, local invasion from these tumors, lung to lung metastasis or synchronous lung tumors was difficult because of limited test results. Unfortunately, we could not investigate other possible factors, such as genetic defects and hormone receptors. But she had no confirmed risk factors, nor did she have trauma or any medical procedure history, she had been in menopause for 2 years and with no recurrence of abdominal mass there was no change in the size and shape of lung nodules during the follow-up after FNAB. Therefore, intrathoracic desmoid tumor can be considered as casual occurrence of other organ caused by uncertain factor, including genetic defect and hormonal factor, rather than metastasis after intra-abdominal desmoid tumor.
In conclusion, we experienced an extremely rare case of true intrathoracic desmoid tumor presenting as multiple lung nodules and occurring 13 years after resection of a previous abdominal wall mass. Although their possible causes are not clear, desmoid tumors rarely occur sequentially in different sites. Because our patient had mild symptoms and no change in imaging tests until now, a wait-and-see policy without additional treatments was followed. Despite improved symptoms and no evidence of disease progression, long-term follow-up would be prudent because of risk of recurrence of her tumor.
Figures and Tables
Figure 2
(A-D) Chest computed tomography scan demonstrating well circumscribed, multiple nodules and multiple ill defined ground glass opacity nodules in both lungs.
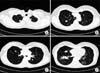
Figure 3
Histologic findings of biopsy. (A) Lung biopsy revealing spindle-shaped myo- fibroblast proliferation within collagenized and myxoid background (H&E stain, ×200). (B) Spindle cells stained with vimentin (×200). (C) Negative for CD34 (×100). (D) The intra-abdominal mass with the same histology as lung biopsy (H&E stain, ×200).

References
1. Dalen BP, Bergh PM, Gunterberg BU. Desmoid tumors: a clinical review of 30 patients with more than 20 years' follow-up. Acta Orthop Scand. 2003; 74:455–459.
2. McKinnon JG, Neifeld JP, Kay S, Parker GA, Foster WC, Lawrence W Jr. Management of desmoid tumors. Surg Gynecol Obstet. 1989; 169:104–106.
3. Posner MC, Shiu MH, Newsome JL, Hajdu SI, Gaynor JJ, Brennan MF. The desmoid tumor. Not a benign disease. Arch Surg. 1989; 124:191–196.
4. Skene AI, Barr L, A'Hern RP, Fisher C, Meirion Thomas J. Multimodality treatment in the control of deep musculoaponeurotic fibromatosis. Br J Surg. 1998; 85:655–658.
5. Bolke E, Krasniqi H, Lammering G, Engers R, Matuschek C, Gripp S, et al. Chest wall and intrathoracic desmoid tumors: surgical experience and review of the literature. Eur J Med Res. 2009; 14:240–243.
6. Andino L, Cagle PT, Murer B, Lu L, Popper HH, Galateau-Salle F, et al. Pleuropulmonary desmoid tumors: immunohistochemical comparison with solitary fibrous tumors and assessment of beta-catenin and cyclin D1 expression. Arch Pathol Lab Med. 2006; 130:1503–1509.
7. Kim NR, Chung DH, Lee JI, Jeong SH, Ha SY. Intrathoracic desmoid tumor mimicking pleural mass: a case report. Tuberc Respir Dis. 2009; 67:449–453.
8. Schwickerath J, Kunzig HJ. Spontaneous malignant transformation of extra-abdominal fibromatosis to fibrosarcoma. Geburtshilfe Frauenheilkd. 1995; 55:173–175.
9. Shields CJ, Winter DC, Kirwan WO, Redmond HP. Desmoid tumours. Eur J Surg Oncol. 2001; 27:701–706.
10. Iqbal M, Rossoff LJ, Kahn L, Lackner RP. Intrathoracic desmoid tumor mimicking primary lung neoplasm. Ann Thorac Surg. 2001; 71:1698–1700.
11. Varghese TK Jr, Gupta R, Yeldandi AV, Sundaresan SR. Desmoid tumor of the chest wall with pleural involvement. Ann Thorac Surg. 2003; 76:937–939.
12. Tejpar S, Nollet F, Li C, Wunder JS, Michils G, dal Cin P, et al. Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor). Oncogene. 1999; 18:6615–6620.
13. Wilcken N, Tattersall MH. Endocrine therapy for desmoid tumors. Cancer. 1991; 68:1384–1388.
14. Briand S, Barbier O, Biau D, Bertrand-Vasseur A, Larousserie F, Anract P, et al. Wait-and-see policy as a first-line management for extra-abdominal desmoid tumors. J Bone Joint Surg Am. 2014; 96:631–638.
15. Aggarwal D, Dalal U, Mohapatra PR, Singhal N. Intra-thoracic desmoid tumor. Lung India. 2012; 29:160–162.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download