Abstract
Non small cell lung cancer (NSCLC) frequently metastasizes to brain, bone, liver, and adrenal glands. While an autopsy of NSCLC reveals some cases of metastasis to the kidney, clinical detection of renal metastases is extremely rare. Furthermore, metastases to the kidney usually present as multifocal or bilateral lesions and solitary renal metastases are usually suspected to be renal cell carcinoma. We now report a case of asymptomatic solitary renal metastasis from a primary squamous cell carcinoma, which was detected by routine surveillance with abdominal CT after curative surgery.
Metastases to the kidney are clinically uncommon in patients with non-small cell lung cancer (NSCLC). However renal metastases have been frequently reported at reviews of analysis for autopsy1,2. Usually in patients with cancer, the renal metastases appear to be multifocal or bilateral tumors and are detected as a part of disseminated malignancy. So, renal cancers to be presented as solitary tumor suggest that they are not metastatic tumors but primary renal cell carcinomas3. In patients with NSCLC, solitary renal metastases have been rarely reported.
Distant metastases in patients with NSCLC are more common in adenocarcinomas compared to the other types. To our knowledge, about 3 cases of solitary renal metastases after curative surgery for squamous cell carcinoma of the lung have been reported in the English literature4-6. Herein, we report a case of solitary renal metastasis from non-small cell lung cancer of which pathologic type was a squamous cell carcinoma.
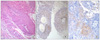
A 65-year-old man was referred for further evaluation of a mass lesion in right lung. He complained of cough and sputum, but denied any other symptom. He was a heavy smoker (60 pack-years) and previously diagnosed with essential hypertension and diabetes mellitus. Chest computed tomography (CT) scans showed a about 3.7×3.4 cm mass in right upper lobe, which compressed right main bronchus and upper lobar bronchus and obstructed right upper lobe apical bronchus (Figure 1). Bronchoscopic biopsy was performed on the lesion and revealed squamous cell carcinoma with well to moderate differentiation. Positron emission tomography-computed tomography (PET-CT) scans showed no abnormal hypermetabolic lesion suggesting regional lymph node metastasis and distant metastasis. Abdomen CT scans showed no evidence of intraabdominal and retroperitoneal metastases. The patient underwent right upper lobectomy and regional lymph node dissection. The tumor size was measured 4.3×3.5 cm. Pathologic TNM stage was T2aN0M0. The patient received adjuvant radiation therapy after curative resection. The patient remained without evidence of disease until 16 months later, when a mass in the kidney was accidently discovered on abdominal CT. The patient did not complain of any symptom and manifest any sign such as hematuria or palpable mass. Laboratory examination did not reveal any abnormal finding such as microscopic hematuria or azotemia. However, abdomen CT scans showed cystic mass with peripheral enhancement on lower pole of right kidney measuring 1.2×1.3 cm (Figure 2). Chest CT scans, bone scan and PET/CT scans revealed no evidence of local recurrence of lung cancer and no other metastatic lesions except solitary renal tumor. Based on clinical and radiological findings, cystic renal cell carcinoma was suspected and he undergone partial nephrectomy. Histological examination of the renal tumor showed a poorly differentiated carcinoma with squamoid differentiation and the tumor cells were positive for p63 and cytokeratin 7 immunostainings (Figure 3). These histologic findings were corresponded with previous findings from primary pulmonary squamous cell carcinoma (Figure 4). He received combination chemotherapy of paclitaxel and cisplatin as 1st line palliative chemotherapy after surgery, but renal metastases were far advanced. Erlotinib was administered as 2nd line but renal metastases did not response. After 2nd line chemotherapy he has received only supportive care.
In this report, we introduced a case of asymptomatic solitary renal metastasis from primary squamous cell carcinoma of lung, which was detected by routine surveillance with abdominal CT after curative surgery. And such pattern of recurrence has been known clinically to be very rare.
The most common sites of distant metastases in patient with NSCLC have been reported to be brain, bone, liver, and adrenal glands7. Clinically, renal metastases from NSCLC are very rare. But, the renal metastases from lung cancer have been reported as a frequent autopsy finding in several autopsy reports1,2. In a large report of 1,000 consecutive postmortem examinations of patients with malignant neoplasms of epithelial origin, Abrams et al. found metastases to the kidney from lung cancer in 22.5% cases1. Two reasons may be considered for such difference between autopsy result and clinical examples. Firstly, in the majority of cases of renal metastases, generally patients rarely complain symptoms as flank pain, palpable mass, or gross hematuria and clinical laboratory tests also show abnormal findings such as hematuria or azotemia3. In the report by Wagle et al.8 none of the patients had gross hematuria and only 31% had microscopic hematuria. Renal failure was presented in only 5% of the reported cases. It is the reason that patients are unable to be screened for renal metastasis from lung cancer. Secondly, it is not generally recommended to screen metastatic tumors to kidney in clinical guidelines for surveillance for lung cancer. In most guidelines, the image studies such as abdomen CT scans or abdomen ultrasonography to be able to find metastatic lesions or relapses below adrenal glands during surveillance are not initially recommended9.
When we searched reports of cases of patients with solitary renal metastasis from squamous cell carcinoma of the lung after curative surgery, about 3 cases have been reported up to recently4-6. In these cases, all patients presented with asymptomatic hematuria and metastatic tumors to the kidney were founded in far advanced status in that they were accompanied by metastases of perirenal lymph nodes. Due to these laboratory abnormal finding, the patients were screened and renal metastasis was confirmed. Differently from such cases, the patient in present case did not complain of any symptom and there was no abnormal laboratory finding such as hematuria or azotemia. A small sized solitary tumor in lower pole of right kidney was accidently found by abdominal CT scans, which was regularly performed during surveillance period after curative surgery for squamous cell carcinoma of the lung. In this case, renal metastasis was found relatively in early status of recurrence and was not accompanied by lymph node metastases.
Recently surgery for resectable metastatic disease or resectable recurrent disease appears to improve survival in patients with cancer. In the treatment for NSCLC, studies of patients with solitary brain metastasis or solitary adrenal metastasis reported a significant survival benefit and surgical resection is strongly recommended for the treatment of such solitary metastatic tumors10,11. It is known that the best treatment for resectable renal cell carcinoma or metastatic renal cancer is surgical resection12. After surgical resection, adjuvant chemotherapy may be performed. The best treatment for solitary renal metastasis from lung cancer, especially squamous cell carcinoma, is not well unknown because such cases are very rare. However, in a few clinical studies of clinical benefit of metastatectomy for resectable metastatic tumors of lung cancer, prognosis of patients with solitary renal metastasis was much poorer than patients with solitary brain metastasis or adrenal gland metastasis13,14. Therefore we think that in the patients with resectable solitary renal metastasis, earlier screening might be helpful for better prognosis.
In present case, the metastatic tumor was located solitary at lower pole of right kidney and was small sized. The spread to regional lymph nodes was not shown through abdominal CT scans, PET/CT scans and intra-operative macroscopic examination. The patient underwent partial nephrectomy for metastatic tumor and thereafter has received cisplatin-based combination chemotherapy.
In summary, solitary renal metastases from squamous cell carcinoma of lung are clinically very rare, while its clinical significance have not been well determined. But because their prognosis is considered to be much poorer than metastatic tumors to brain or adrenal glands, we think that early detection during surveillance may be of assistance to improve survival of the patients.
Figures and Tables
Figure 1
Computed tomographic scan of the chest shows a tumor in the right upper lobe with invasion and compression of the right main bronchus 2cm or more from the carina (white arrow).
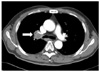
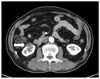
Figure 2
Computed tomographic scan of the abdomen shows a cystic mass with peripheral enhancement in the right kidney (white arrow).
References
1. Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950. 3:74–85.
2. Hietala SO, Wahlqvist L. Metastatic tumors to the kidney. A postmortem, radiologic and clinical investigation. Acta Radiol Diagn (Stockh). 1982. 23:585–591.
3. Bailey JE, Roubidoux MA, Dunnick NR. Secondary renal neoplasms. Abdom Imaging. 1998. 23:266–274.
4. Akduman B, Altun R, Yesilli C, Yenidunya S, Ozdemir H, Mungan NA. Symptomatic renal metastasis 5 years after the management of a squamous cell carcinoma of the lung. Int J Urol. 2004. 11:421–423.
5. Fujimoto K, Ozono S, Okamoto S, Tsujimoto S, Oyama N, Yamada H, et al. A case of metastatic renal tumor: review of 74 cases reported in Japan. Hinyokika Kiyo. 1990. 36:581–585.
6. Sugiyama T, Tsujihashi H, Matsuura T, Kaneko S, Kohri K, Akiyama T, et al. Metastatic renal tumor. Hinyokika Kiyo. 1983. 29:1499–1505.
7. Quint LE, Tummala S, Brisson LJ, Francis IR, Krupnick AS, Kazerooni EA, et al. Distribution of distant metastases from newly diagnosed non-small cell lung cancer. Ann Thorac Surg. 1996. 62:246–250.
8. Wagle DG, Moore RH, Murphy GP. Secondary carcinomas of the kidney. J Urol. 1975. 114:30–32.
9. Rubins J, Unger M, Colice GL. American College of Chest Physicians. Follow-up and surveillance of the lung cancer patient following curative intent therapy: ACCP evidence-based clinical practice guideline (2nd edition). Chest. 2007. 132:3 Suppl. 355S–367S.
10. Hu C, Chang EL, Hassenbusch SJ 3rd, Allen PK, Woo SY, Mahajan A, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer. 2006. 106:1998–2004.
11. Mercier O, Fadel E, de Perrot M, Mussot S, Stella F, Chapelier A, et al. Surgical treatment of solitary adrenal metastasis from non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005. 130:136–140.
12. Nabi G, Cleves A, Shelley M. Surgical management of localised renal cell carcinoma. Cochrane Database Syst Rev. 2010. (3):CD006579.
13. Ambrogi V, Tonini G, Mineo TC. Prolonged survival after extracranial metastasectomy from synchronous resectable lung cancer. Ann Surg Oncol. 2001. 8:663–666.
14. Yoshino I, Yohena T, Kitajima M, Ushijima C, Nishioka K, Ichinose Y, et al. Survival of non-small cell lung cancer patients with postoperative recurrence at distant organs. Ann Thorac Cardiovasc Surg. 2001. 7:204–209.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download